Structural Characterization of Peripolin and Study of Antioxidant Activity of HMG Flavonoids from Bergamot Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Standards and Bergamot Samples
2.2. Purification and Characterization of Peripolin
2.2.1. Sample Preparation
2.2.2. Fractionation and Purification of Peripolin by Semipreparative HPLC-UV
2.2.3. HPLC/UV-MS Analysis of Fractions and Tissues Extracts
2.2.4. High-Resolution Mass Spectrometry
2.2.5. Nuclear Magnetic Resonance
2.2.6. Sample Preparation for UPLC-MS/MS Assay
2.2.7. UPLC-MS/MS Assay
2.3. Antioxidant Capacity Assays
2.3.1. DPPH Assay
2.3.2. ABTS Assay
2.3.3. FRAP Assay
2.4. Computational Details
3. Results
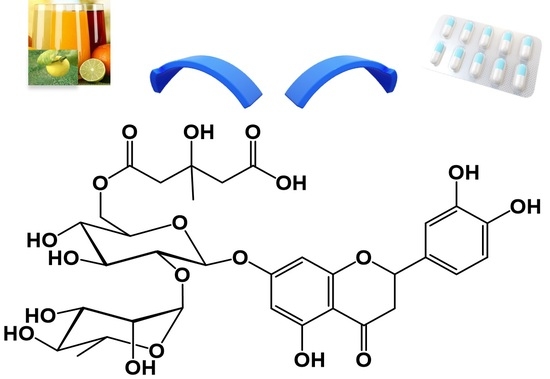
3.1. Structural Characterization of Peripolin
3.2. Antioxidant Activity of HMG Flavonoids
3.3. Computational Studies of the Antioxidant Capacity of HMG Flavonoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.fao.org/3/cb6492en/cb6492en.pdf (accessed on 16 July 2022).
- Giuffrè, A.M. Bergamot (Citrus bergamia, Risso): The effects of cultivar and harvest date on functional properties of juice and cloudy juice. Antioxidants 2019, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M.; Nobile, R. Citrus bergamia, risso: The peel, the juice and the seed oil of the bergamot fruit of Reggio Calabria (south Italy). Emir. J. Food Agr. 2020, 32, 522–532. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Distribution of flavonoids and furocoumarins in juices from cultivars of citrus bergamia risso. J. Agr. Food Chem. 2007, 55, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Gupta, S.; Ojha, S.K.; Sharma, S.B. Cardiovascular friendly natural products: A promising approach in the management of CVD. Nat. Prod. Res. 2010, 24, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Cruz-Martins, N.; Butnariu, M.; Sarac, I.; Bagiu, I.C.; Ezzat, S.M.; Wang, J.; Koay, A.; Sheridan, H.; Adetunji, C.O.; et al. Hesperetin’s health potential: Moving from preclinical to clinical evidence and bioavailability issues, to upcoming strategies to overcome current limitations. Crit. Rev. Food Sci. Nutr. 2021; in press. [Google Scholar] [CrossRef]
- Gattuso, G.; Caristi, C.; Gargiulli, C.; Bellocco, E.; Toscano, G.; Leuzzi, U. Flavonoid glycosides in bergamot juice (citrus bergamia risso). J. Agr. Food Chem. 2006, 54, 3929–3935. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Vrablik, M.; Al Rasadi, K.; Banach, M.; Toth, P.P.; Rizzo, M. Nutraceuticals in the management of dyslipidemia: Which, when, and for whom? Could nutraceuticals help low-risk individuals with non-optimal lipid levels? Curr. Atheroscleros. Rep. 2021, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Lamiquiz-Moneo, I.; Giné-González, J.; Alisente, S.; Bea, A.M.; Pérez-Calahorra, S.; Marco-Benedí, V.; Baila-Rueda, L.; Jarauta, E.; Cenarro, A.; Civeira, F.; et al. Effect of bergamot on lipid profile in humans: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3133–3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Donna, L.; De Luca, G.; Mazzotti, F.; Napoli, A.; Salerno, R.; Taverna, D.; Sindona, G. Statin-like principles of bergamot fruit (Citrus bergamia): Isolation of 3-hydroxymethylglutaryl flavonoid glycosides. J. Nat. Prod. 2009, 72, 1352–1354. [Google Scholar] [CrossRef]
- Di Donna, L.; Iacopetta, D.; Cappello, A.R.; Gallucci, G.; Martello, E.; Fiorillo, M.; Dolce, V.; Sindona, G. Hypocholesterolaemic activity of 3-hydroxy-3-methyl-glutaryl flavanones enriched fraction from bergamot fruit (Citrus bergamia): ‘In vivo’ studies. J. Funct. Food. 2014, 7, 558–568. [Google Scholar] [CrossRef]
- Di Donna, L.; Gallucci, G.; Malaj, N.; Romano, E.; Tagarelli, A.; Sindona, G. Recycling of industrial essential oil waste: Brutieridin and melitidin, two anticholesterolaemic active principles from bergamot albedo. Food Chem. 2011, 125, 438–441. [Google Scholar] [CrossRef]
- Yamada, M.; Tanabe, F.; Arai, N.; Mitsuzumi, H.; Miwa, Y.; Kubota, M.; Chaen, H.; Kibata, M. Bioavailability of glucosyl hesperidin in rats. Biosci Biotechnol Biochem. 2006, 70, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Ávila-gálvez, M.Á.; Giménez-bastida, J.A.; González-sarrías, A.; Espín, J.C. New insights into the metabolism of the flavanones eriocitrin and hesperidin: A comparative human pharmacokinetic study. Antioxidants 2021, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- ESCO Working Group on Botanicals and Botanical Preparations. EFSA scientific cooperation (ESCO) report. Advice on the EFSA guidance document for the safety assessment of botanicals and botanical preparations intended for use as food supplements, based on real case studies. EFSA J. 2009, 7, 280. [Google Scholar] [CrossRef]
- Mazzotti, F.; Bartella, L.; Talarico, I.R.; Napoli, A.; Di Donna, L. High-throughput determination of flavanone-O-glycosides in citrus beverages by paper spray tandem mass spectrometry. Food Chem. 2021, 360, 130060. [Google Scholar] [CrossRef] [PubMed]
- Salerno, R.; Casale, F.; Calandruccio, C.; Procopio, A. Characterization of flavonoids in citrus bergamia (bergamot) polyphenolic fraction by liquid chromatography–high resolution mass spectrometry (LC/HRMS). PharmaNutrition 2016, 4, S1–S7. [Google Scholar] [CrossRef]
- Di Donna, L.; Dolce, V.; Sindona, G. Patent Nr. WO2010041290, A1·2010-04-15. 2010. Available online: https://worldwide.espacenet.com/patent/search/family/040599649/publication/WO2010041290A1?q=WO2010041290A1 (accessed on 30 July 2022).
- Pyrzynska, K.; Pekal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 2013, 5, 4288. [Google Scholar] [CrossRef]
- Bartella, L.; Mazzotti, F.; Talarico, I.R.; Santoro, I.; Di Donna, L. Hydroxytyrosol-fortified foods obtained by supercritical fluid extraction of olive oil. Antioxidants 2021, 10, 1619. [Google Scholar] [CrossRef]
- Formagio, A.S.; Volobuff, C.R.; Santiago, M.; Cardoso, C.A.; Vieira, M.; Valdevina Pereira, Z. Evaluation of Antioxidant Activity, Total Flavonoids, Tannins and Phenolic Compounds in Psychotria Leaf Extracts. Antioxidants 2014, 3, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power: The FRAP Assay”. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Fox, D.J. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Prejanò, M.; Romeo, I.; Sgrizzi, L.; Russo, N.; Marino, T. Why hydroxy-proline improves the catalytic power of the peptidoglycan: N-deacetylase enzyme: Insight from theory. Phys. Chem. Chem. Phys. 2019, 21, 23338–23345. [Google Scholar] [CrossRef]
- Pérez-González, A.; Prejanò, M.; Russo, N.; Marino, T.; Galano, A. Capsaicin, a Powerful •OH-Inactivating Ligand. Antioxidants 2020, 9, 1247. [Google Scholar] [CrossRef]
- Prejanò, M.; Romeo, I.; Russo, N.; Marino, T. On the Catalytic Activity of the Engineered Coiled-Coil Heptamer Mimicking the Hydrolase Enzymes: Insights from a Computational Study. Int. J. Mol. Sci. 2020, 21, 4551. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Kollman, P.A. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Phys. Chem. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Marino, T.; Prejanò, M.; Russo, N. On the Scavenging Ability of Scutellarein against the OOH Radical in Water and Lipid-like Environments: A Theoretical Study. Antioxidants 2022, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Marino, T.; Prejanò, M.; Russo, N.; Toscano, M. Antioxidant Properties of the Vam3 Derivative of Resveratrol. Molecules 2018, 23, 2446. [Google Scholar] [CrossRef]
- Parise, A.; De Simone, B.C.; Marino, T.; Toscano, M.; Russo, N. Quantum Mechanical Predictions of the Antioxidant Capability of Moracin C Isomers. Front. Chem. 2021, 9, 666647. [Google Scholar] [CrossRef]
- Malacaria, L.; La Torre, C.; Furia, E.; Fazio, A.; Caroleo, M.C.; Cione, E.; Gallelli, L.; Marino, T.; Plastina, P. Aluminum(III), iron(III) and copper(II) complexes of luteolin: Stability, antioxidant, and anti-inflammatory properties. J. Mol. Liq. 2022, 345, 117895. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species, Biosci. Biotech. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Available online: http://www.system24.ilsole24ore.com/static/minisiti/2014/bside/201014_SUD_CHIMICA_FARMACEUTICA/Pagine/3.pdf (accessed on 16 July 2022).
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Ma, Y.L.; Li, Q.M.; Van den Heuvel, H.; Claeys, M. Characterization of Flavone and Flavonol Aglycones by Collision-induced Dissociation Tandem Mass Spectrometry. Rapid. Commun. Mass Spectrom. 1997, 11, 1357–1364. [Google Scholar] [CrossRef]
- Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32012R0432 (accessed on 14 August 2022).






| Peripolin (1) | ||
|---|---|---|
| Position | δH (J in Hz) | δC Mult |
| 2 | 5.34 dd (2.90, 12.7) | 80.6, CH |
| 3a | 3.12 dd (12.7, 17.2) | 44.0, CH2 |
| 3b | 2.74 dd (2.9, 17.2) | 44.3, CH2 |
| 4 | 198.4, qC | |
| 5 | 164.9, qC | |
| 6 | 6.16 m, ar | 97.0, CH |
| 7 | 166.5, qC | |
| 8 | 6.14 m, ar | 98.1, CH |
| 9 | 164.6, qC | |
| 10 | 105.1. qC | |
| 1′ | 131.7, qC | |
| 2′ | 6.79 m, ar | 116.5, CH |
| 3′ | 147.0, qC | |
| 4′ | 146.6, qC | |
| 5′ | 6.80 m, ar | 119.4, CH |
| 6′ | 6.93 m, ar | 114.9, CH |
| 1″ | 5.08 d (7.6) | 99.5, CH |
| 2″ | 3.66 dd (7.7, 9.0) | 78.9, CH |
| 3″ | 3.60 dd (8.3, 9.0) | 79.1, CH |
| 4″ | 3.36 dd (8.3, 10.4) | 71.8, CH |
| 5″ | 3.69 ddd (2.0, 7.5, 11.9) | 75.5, CH |
| 6″a | 4.40 dd (2.0, 11.9) | 64.6, CH2 |
| 6″b | 4.19 dd (7.5, 11.9) | |
| 1‴ | 5.25 d (1.4) | 102.5, CH |
| 2‴ | 3.93 dd (1.4, 3.2) | 72.2, CH |
| 3‴ | 3.0 m | 72.3, CH |
| 4‴ | 3.40 dd (1.6, 9.5) | 74.1, CH |
| 5‴ | 3.90 ddd (3.9, 6.2, 9.5) | 70.0, CH |
| 6‴ | 1.30 d (6.2) | 18.2, CH3 |
| 1⁗ | 172.5, qC | |
| 2⁗ | 2.72–2.52 m | 45.0, CH2 |
| 3⁗ | 70.7, qC | |
| 4⁗ | 2.72–2.52 m | 46.5, CH2 |
| 5⁗ | 174.9, qC | |
| 6⁗ | 1.27 s | 27.7, CH3 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| PE1 ‡ | 6.0 ± 0.1 ‡ | 40 ± 1 ‡ | 28 ± 1 ‡ | 164 ± 3 ‡ | 160 ± 9 ‡ | 78 ± 4 ‡ |
| PE2 ‡ | 5.0 ± 0.7 ‡ | 39 ± 4 ‡ | 32 ± 4 ‡ | 152 ± 4 ‡ | 129 ± 5 ‡ | 71 ± 2 ‡ |
| PE3 ‡ | 4.2 ± 0.3 ‡ | 29.0 ± 0.2 ‡ | 15 ± 2 ‡ | 138 ±7 ‡ | 130 ± 3 ‡ | 85 ± 1 ‡ |
| PE4 ‡ | 3.1 ± 0.2 ‡ | 28 ± 1 ‡ | 16 ± 1 ‡ | 136 ± 6 ‡ | 120 ± 8 ‡ | 85 ± 5 ‡ |
| PE5 ‡ | 3.0 ± 0.2 ‡ | 28 ± 1 ‡ | 16 ± 1 ‡ | 145 ± 5 ‡ | 119 ± 9 ‡ | 90 ± 5 ‡ |
| SB1 * (30% bergamot juice) | 23.5± 0.1 * | 53.1 ± 0.1 | 29.6± 0.2 | 76.7 ± 0.3 * | 50.4 ± 0.2 * | 57.8 ± 0.2 * |
| PB1 * (Stevia and 30% bergamot juice) | 13.7 ± 0.6 * | 68.4 ± 0.3 * | 36.5 ± 0.2 * | 106.1 ± 2 * | 53.6 ± 0.2 * | 79.1 ± 0.3 * |
| SB2 * (100% bergamot juice 2019) | 56.1± 0.2 * | 184 ± 2 * | 66 ± 0.3 * | 678 ± 13 * | 611 ± 22 * | 503 ± 7 * |
| SB3 * (100% bergamot juice 2020) | 66.2 ± 0.1 * | 187 ± 4 * | 92 ± 0.1 * | 354 ± 14 * | 234± 2 * | 334 ± 12 * |
| SB4 * (100% bergamot juice 2021) | 108.1 ± 2 * | 380 ± 12 * | 162 ± 11 * | 617 ± 13 * | 332 ± 8 * | 520 ± 17 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartella, L.; Mazzotti, F.; Talarico, I.R.; De Luca, G.; Santoro, I.; Prejanò, M.; Riccioni, C.; Marino, T.; Di Donna, L. Structural Characterization of Peripolin and Study of Antioxidant Activity of HMG Flavonoids from Bergamot Fruit. Antioxidants 2022, 11, 1847. https://doi.org/10.3390/antiox11101847
Bartella L, Mazzotti F, Talarico IR, De Luca G, Santoro I, Prejanò M, Riccioni C, Marino T, Di Donna L. Structural Characterization of Peripolin and Study of Antioxidant Activity of HMG Flavonoids from Bergamot Fruit. Antioxidants. 2022; 11(10):1847. https://doi.org/10.3390/antiox11101847
Chicago/Turabian StyleBartella, Lucia, Fabio Mazzotti, Ines Rosita Talarico, Giuseppina De Luca, Ilaria Santoro, Mario Prejanò, Costanza Riccioni, Tiziana Marino, and Leonardo Di Donna. 2022. "Structural Characterization of Peripolin and Study of Antioxidant Activity of HMG Flavonoids from Bergamot Fruit" Antioxidants 11, no. 10: 1847. https://doi.org/10.3390/antiox11101847