Abstract
Summary
Denosumab leads to improvements in BMD levels and is a well-tolerated agent according to results of randomized controlled studies but results in real-life setting are important to evaluate drug adherence and real-life efficiency. In this study, we present the results of 305 patients that were treated with denosumab in our clinic.
Introduction
The long-term efficacy of anti-osteoclastic drugs in treatment of osteoporosis is well known. Denosumab, a novel human monoclonal antibody, is an anti-osteoclastic agent that has been shown to lead to reductions in vertebral, nonvertebral, and hip fracture risk in randomized and observational studies. Real-life data of this agent is increasing. In this study, we presented our real-life data about the 2-year follow-up of patients under denosumab treatment.
Methods
Osteoporotic patients who were treated with at least one denosumab injection between 2014 and 2020 years were included. Clinical and demographic data, bone turnover markers, and radiological reports (bone mineral densitometry (BMD), vertebral x-ray) were obtained from patient files retrospectively.
Results
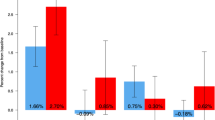
A total of 305 patients (f/m: 275/30, 68.1 ± 11.05 years) were included. The median injection number was 4 (1–10). Two hundred seventy-three patients (89.8%) were persistent on treatment at the 12th month; 175 patients (57.3%) were persistent at 24th month. Sixty-eight patients (22%) were not using denosumab anymore, 55 of the patients were not continuing by doctor desicion and 13 were not continuing due to patient-related causes. Median BMD levels significantly increased from 0.809 (0.2–1.601, IQR: 0.136) to 0.861 (0.517–1.607, IQR: 0.14) in L1–L4 and from 0.702 (0.349–0.997, IQR: 0.125) to 0.745 (0.508–1.008, IQR: 0.137) in femur area at the 24th month of treatment. An improvement of 8.04% in L1–L4 BMD and 4.5% in femur neck BMD levels at the 24th month of treatment was observed. There was a significant decrease in bone turnover markers at the 24th month of treatment.
Conclusion
In our group of patients under denosumab treatment, 53% of persistence was found at 24 months and associated with improvement in BMD levels without any significant side effects except one case with urticarial reaction. Denosumab leads to improvements in BMD levels and is a well-tolerated agent in a real-life setting comparable to results of randomized controlled studies in patients with different comorbidities.



Similar content being viewed by others
Data availability
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.
References
Hannan E, Magaziner J, Wang J et al (2001) Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes. JAMA 285:2736–2742
Cooper C, Atkinson EJ, Jacobsen SJ (1993) Population-bpased study of survival after osteoporotic fractures. Am J Epidemiol 137:1001–1005
Roux C, Wyman A, Hooven F et al (2012) Burden of non-hip, nonvertebral fractures on quality of life in postmenopausal women. Osteoporos Int 23(12):2863–2871
US Department of Health and Human Services. Bone health and osteoporosis: a report of the surgeon general. US Department of Health and Human Services, Office of the Surgeon General; 2004.
Kothawala P, Badamgarav E, Ryu S et al (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82(12):1493–1501
Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ et al (2003) Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 290:1729–1738
McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L et al (2013) Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 126:13–20
Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L et al (2007) Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 39:960–962
Goessl C, Katz L, Dougall WC, Kostenuik PJ, Zoog HB, Braun A, Dansey R, Wagman RB (2012) The development of denosumab for the treatment of diseases of bone loss and cancer-induced bone destruction. Ann N Y Acad Sci 1263:29–40. https://doi.org/10.1111/j.1749-6632.2012.06674.x
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med, 361:756–765
Papapoulos S, Lippuner K, Roux C et al (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int 26(12):2773–2783. https://doi.org/10.1007/s00198-015-3234-7
Zaheer S, LeBoff M, Lewiecki EM (2015) Denosumab for the treatment of osteoporosis. Expert Opin Drug Metab Toxicol 11(3):461–470. https://doi.org/10.1517/17425255.2015.1000860
Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP (2018) Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Min Res 33(2):190–198
Tsourdi E, Zillikens MC, Meier C, Body JJ, Rodriguez EG, Anastasilakis AD, Abrahamsen B, McCloskey E, Hofbauer LC, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Pepe J, Palermo A, Langdahl B. (2020) Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 26:dgaa756. https://doi.org/10.1210/clinem/dgaa756
Freemantle N, Satram-Hoang A, Tang E et al (2012) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23(1):317–326
Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9(8):1137–1141
Moran CP, English S, Beringer T, Lindsay JR (2019) Real world experience of denosumab treatment in the Belfast Osteoporosis Service. Ulster Med J 88(3):150–156
Morley J, Moayyeri A, Ali L, Taylor A, Feudjo-Tepie M, Hamilton L, Bayly J (2020) Persistence and compliance with osteoporosis therapies among postmenopausal women in the UK Clinical Practice Research Datalink. Osteoporos Int 31(3):533–545. https://doi.org/10.1007/s00198-019-05228-8
Tremblay É, Perreault S, Dorais M (2016) Persistence with denosumab and zoledronic acid among older women: a population-based cohort study. Arch Osteoporos 11(1):30. https://doi.org/10.1007/s11657-016-0282-3
Boschitsch E, Naegele O, Klinger A, Samoylenko H. Long-term persistence with denosumab: real-world data from the Austrian Osteoporosis Clinic (AOC). A retrospective data analysis
Růžičková O, Killinger Z, Kasalický P et al (2018) Real-world management of women with postmenopausal osteoporosis treated with denosumab: a prospective observational study in the Czech Republic and Slovakia. Adv Ther 35(10):1713–1728. https://doi.org/10.1007/s12325-018-0779-9
Gutiérrez-Fernández D, Cruz MJ, Foncubierta-Fernández A et al (2015) Monoclonal antibody desensitization in a patient with a generalized urticarial reaction following denosumab administration. Allergy Asthma Clin Immunol 11:29. Published 2015 Oct 26. https://doi.org/10.1186/s13223-015-0097-6
E C-C Lai, Lin, T-C Lin, J L Lange et al (2022) Effectiveness of denosumab for fracture prevention in real-world postmenopausal women with osteoporosis: a retrospective cohort study. Osteoporos Int 33. https://doi.org/10.1007/s00198-021-06291-w
Thongprayoon C, Acharya P, Aeddula NR, Torres-Ortiz A, Bathini T, Sharma K, Ungprasert P, Watthanasuntorn K, Suarez MLG, Salim SA, Kaewput W, Chenbhanich J, Mao MA, Cheungpasitporn W (2019) Effects of denosumab on bone metabolism and bone mineral density in kidney transplant patients: a systematic review and meta-analysis. Arch Osteoporos 14(1):35. https://doi.org/10.1007/s11657-019-0587-0.PMID:30852679.BottomofForm
Thongprayoon C, Acharya P, Acharya C, Chenbhanich J, Bathini T, Boonpheng B, Sharma K, Wijarnpreecha K, Ungprasert P, Gonzalez Suarez ML, Cheungpasitporn W (2018) Hypocalcemia and bone mineral density changes following denosumab treatment in end-stage renal disease patients: a meta-analysis of observational studies. Osteoporos Int 29(8):1737–1745. https://doi.org/10.1007/s00198-018-4533-6
Silverman SL, Siris E, Belazi D et al (2018) Persistence at 24 months with denosumab among postmenopausal women with osteoporosis: results of a prospective cohort study. Arch Osteoporos 13(1):85. Published 2018 Aug 7. https://doi.org/10.1007/s11657-018-0491-z
Acknowledgements
All authors read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yazan, C.D., Bugdayci, O., Ilgin, C. et al. Effect of denosumab treatment on bone mineral density and bone turnover markers in osteoporotic patients: real-life experience 2-year follow-up. Arch Osteoporos 17, 125 (2022). https://doi.org/10.1007/s11657-022-01145-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-022-01145-2