Abstract
COVID-19 patients in critical conditions are hospitalized and treated with various protocols including antiviral drugs, which have been updated repeatedly. This study was aimed to analyze the demographics, costs, and outcomes of drug regimens in COVID-19 patients hospitalized in “Ali Asghar” hospital, affiliated with Shiraz University of Medical Sciences, from March 2019 to December 2020 as a retrospective study, approved by the ethics committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1399.1003) on Dec. 28, 2020. Using hospital information system (HIS) data, 2174 patients receiving favipiravir, remdesivir, interferon-β, and Kaletra® were analyzed. Descriptive, univariate, and regression analyses were used. The costs and consequences of different drug regimens were significantly different (P value < 0.05); the highest and lowest costs belonged to remdesivir and Kaletra®, respectively. The highest and lowest mean length of stay and mortality were related to remdesivir and favipiravir, respectively. Mortality did not differ significantly with various regimens. Length of stay was significantly shorter with favipiravir and Kaletra® than interferon-β. Remdesivir had significantly the highest cost. Age presented a significantly positive relationship with mortality and length of stay. Besides, ICU admission significantly increased mortality, length of stay, and costs. Underlying diseases and low blood oxygen saturation contributed to mortality. COVID-19 correlation with age and underlying diseases is accordant with the published data. Given the highest costs and broad usage of remdesivir, besides controversies regarding its outcomes and side effects, a stricter evaluation of remdesivir benefits seems essential. Totally, COVID-19 therapeutic protocols should be selected carefully to optimize costs and outcomes.
Similar content being viewed by others
1 Introduction
For the third time in recent decades, a species of coronaviruses invaded humans, named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was first identified in Wuhan, China, in December 2019 (Perlman 2020) and rapidly led to a pandemic. According to the Worldmeter, more than 284 million individuals have been infected with COVID-19 globally by February 2022, with more than 5.7 million cases of related mortality (https://www.worldometers.info/coronavirus/, accessed 21 December 2021). Coronaviruses include four generations named as α-, β-, γ-, and δ-coronaviruses, whereas α- and β-coronaviruses infect mammals. Human coronaviruses may cause Middle East respiratory syndrome coronavirus (MERS-CoV), Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), and SARS-CoV-2 (Shen et al. 2020; V'Kovski et al. 2021; Li et al. 2020).
SARS-CoV-2 is an enveloped RNA virus. Its pathogenesis involves viral proliferation in lower respiratory tract epithelial cells, similar to SARS-CoV and MERS-CoV. However, it is more fatal than the other two mentioned species. SARS-CoV-2 employs human cells’ facilities leading to replication of its RNA to make their own proteins, including nucleocapsid (N), spike glycoprotein (S), envelope (E), and membrane (M) proteins (Martines et al. 2020; Lotfi and Rezaei 2020). The spike protein is essential for interaction between the virus and cell receptors during viral entry to human cells. Similar to SARS-CoV, SARS-CoV-2 enters human cells by the S protein binding to the ACE2 receptors. It can also enter the cell through clathrin-mediated endocytosis (Owji et al. 2020).
Therefore, the proteins mentioned above are known as significant targets for antiviral agents against SARS-CoV and MERS-CoV. Given the 79.5% identity between the SARS-CoV-2 and SARS-CoV genomes, it was suggested that the medications previously used in SARS-CoV epidemics, such as galidesivir, ribavirin, lopinavir/ritonavir, remdesivir, and interferon (IFN)-α and -β may also be effective against SARS-CoV-2 (Lotfi and Rezaei 2020; Elshabrawy 2020). Thus, previous antiviral drugs and many other drugs were alternately suggested for COVID-19 treatment. However, many of them did not prove to be effective in clinical trials (Negahdaripour 2020).
In general, the therapeutic protocol for COVID-19 depends on the patient's condition. The patients with suspected and confirmed COVID-19 should be self-isolated and receive supportive and symptomatic treatments. However, those in critical conditions should be hospitalized and treated with antiviral drugs (Shen et al. 2020; Srinivas et al. 2020).
IFN-β is a medication suggested for treating COVID-19 (Bagheri et al. 2021). According to several studies, IFNs are highly important in the effectiveness of adaptive immune responses against viral infections at various stages (Mosaddeghi et al. 2021; http://corona.behdasht.gov.ir/, accessed 13 November 2021). IFN-β can reduce the serum viral loads leading to symptom alleviation in the early stages of the disease (Shen et al. 2020). On the other hand, elevated IFN levels in the advanced stages of COVID-19 may delay the recovery and increase the disease fatality by inducing the ACE2 overexpression in the human respiratory tract epithelial cells, which facilitates viral entry into host cells (Parashkouhi et al. 2021). This drug has been investigated in several clinical studies. A study using inhalatory IFN-β1a in 48 COVID-19 patients reported higher therapeutic progress than placebo on days 15 and 16 (Monk et al. 2021). Another study by Park and Iwasaki found reduced responses mediated by IFNs in COVID-19 patients. Additionally, the antiviral effects of IFN have been confirmed in several other in vitro and in vivo studies (Park and Iwasaki 2020).
Remdesivir is the first medication approved by the US FDA as well as the European Union regulatory authorities for clinical use in hospitalized patients with COVID-19 (https://www.ncbi.nlm.nih.gov/books/NBK563261/, accessed December 2021; Wise 2020). Remdesivir is an adenosine analog prodrug inhibiting the viral RNA-dependent RNA polymerase. It has shown satisfactory in vitro results in SARS-CoV and MERS-CoV (Beigel et al. 2020; Doggrell 2020). A multicenter randomized clinical trial reported its higher effects than placebo in reducing the hospital stay of COVID-19 patients (Beigel et al. 2020; Negahdaripour 2020).
Favipiravir, another antiviral drug suggested for COVID-19 treatment (Sanchez-Felipe et al. 2020), is a viral RNA-dependent RNA polymerase previously used in Japan to treat Influenza caused by influenza virus novel strains (Yamamura et al. 2020). Another candidate drug was the combination of lopinavir/ritonavir (Kaletra®), which is a protease inhibitor mainly used for AIDS treatment (Walmsley et al. 2002). However, it was removed from the AIDS therapeutic protocol by the world health organization (WHO) due to its slight effect on reducing the related mortality (Negahdaripour 2020).
According to the studies, the direct COVID-19 treatment costs are significantly higher than other common infectious diseases, because this disease is associated with a higher chance of hospitalization and mortality (Bartsch et al. 2020). Moreover, a significant proportion of the patients with COVID-19 require expensive intensive care services due to their severe disease. Therefore, the therapeutic costs for COVID-19 are remarkable (Molina et al. 2014).
The objectives of the present study were to compare the costs, outcomes, and effectiveness of different drug regimens used for COVID-19 treatment, including favipiravir, remdesivir, IFN-β, and Kaletra®, and evaluate the effects of demographic and clinical factors in this disease.
2 Methods
2.1 Study Design and Data Collection
The present historical cohort study was a partial evaluation of the cost-outcome description or Cost-Consequence Analysis (CCA) type. It was approved by the ethics committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1399.1003). Data collection included the clinical outcomes, treatment costs, underlying diseases, and demographics of the COVID-19 patients who were treated with favipiravir, remdesivir, IFN-β, and Kaletra® in the Ali Asghar Hospital affiliated with Shiraz University of Medical Sciences from March 2019 to December 2020. The data were collected from the hospital information system (HIS) of the mentioned hospital. It should be noted that in the current study, only medicines that had been prescribed in the hospital were analyzed, so self-medication was not included. Noting that hospitalized patients do not take medicines outside the prescribed medicines.
The costs were converted to US dollars using a purchasing power parity (PPP) $ exchange rate of 30,007 Rials per 1PPP$ in 2020 (World Bank 2020; Singh 2020).
2.2 Study Population
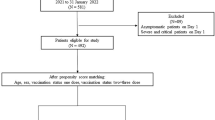
The study population included all the patients who referred to Ali Asghar Hospital, a referral hospital for COVID-19 in Shiraz, Iran, with COVID-19 from the beginning of February 2020 to the end of November 2021, and their treatment course, from diagnosis to discharge, was entirely performed in the mentioned hospital. The study used the census method. Therefore, there was no sampling. Overall, more than 3000 patients were admitted to Ali Asghar Hospital due to COVID-19 from the beginning of March 2019 to December 2020. After data cleaning, 2174 patients were included in the study.
The study tool included a researcher-made form consisting of the patients' demographics, clinical outcomes, and therapeutic costs. The forms were filled using the information available in the medical records of the patients and their medical bills available in the accounting unit of the hospital. In general, medical costs can be divided into two groups: direct costs and indirect costs. Given the present study view, which was the view of the Ministry of Health and Medical Education of Iran, and the present limitations, only direct treatment costs were considered. Therefore, the direct therapeutic and diagnostic costs, including physician visits, laboratory investigations, imaging services, medications, the material used, oxygen administration, and capital goods, were collected for the patients receiving each drug regimen. The primary therapeutic outcomes included mortality and hospital stay.
2.3 Data Analysis
This study first presented the descriptive statistics of all the participants based on the costs and outcomes of different drug regimens, demographic variables, and main factors related to COVID-19. Afterward, the differences in costs (medication costs and total treatment costs) and outcomes (hospital stay and mortality) were investigated in different drug regimens. In an univariate analysis, the Kruskal–Wallis test was used to investigate the mean differences in costs, hospital stay, and mortality among the patients receiving different drug regimens.
Finally, the regression analysis was used to investigate the effect of different drug regimens on the costs and outcomes considering demographic and clinical variables. Moreover, linear multivariate regression and ordinary least squares estimation were used for cost and hospital stay models, as well as the logistic regression and maximum likelihood estimation for the mortality model. The explanatory variables in all models included the drug regimen (favipiravir, remdesivir, IFN-β, and Kaletra®), drug cost, total treatment cost, hospital stay, age, gender, ICU admission, underlying diseases, mortality, and oxygen saturation. Likelihood ratio (LR) chi-square test and F-statistic were used for models' goodness of fit.
3 Results
The demographic and clinical characteristics of the patients are presented in Table 1. According to our results, 19.6% of the patients were younger than 40 years old, 51.6% aged 40–65 years old, and 28.8% were older than 65. Moreover, about 55.6% were female, and 44.4% were male. Besides, 207 patients (9.5%) were expired during hospitalization.
In terms of medication costs, the drug regimen costs were less than 10 million Rials (333 $PPP) for 44.2% of the patients, while the mentioned costs were 35–45 million Rials (1167–1500 $PPP) for 47.7% of the patients. Moreover, 8.2% of the patients had a drug cost of more than 45 million Rials (1500 $PPP). Moreover, the total treatment costs were < 50 (1667 $PPP), 50–100 (1667–3333 $PPP), 100–200 (3333–6667 $PPP), and > 200 (6667 $PPP) million Rials for 32.8, 38.6, 17.4, and 11.2% of the patients, respectively.
In terms of hospital stay, 19.6% of the patients had a hospital stay of fewer than 40 days, 51.6% were hospitalized for 40–65 days, and 28.8% were in the hospital for more than 65 days. Finally, IFN-β, favipiravir, remdesivir, and Kaletra® were administered for 13.9, 4.3, 55.8, and 25.8% of the patients. Additionally, 9.4% of the patients were ICU-admitted.
The mean differences in costs, hospital stay, and mortality between the patients receiving different drug regimens and the related results are presented in Table 2. According to our findings, drug regimens had significant effects on the costs and outcomes of treatment (all P values < 0.05). The patients receiving Kaletra® had the lowest drug regimen costs, while those receiving remdesivir had the highest costs. Moreover, the patients receiving favipiravir had the shortest hospital stay and lowest mortality, while those receiving remdesivir had the highest mortality and longest hospital stay.
The co-effect of demographics and drug regimens on mortality, hospital stay, and treatment costs were investigated using the multivariate regression model, and the related results are presented in Table 3.
In terms of mortality, the present study results showed that COVID-19-related mortality was higher in older patients than younger ones. Moreover, the ICU-admitted patients were 115 times more likely to die due to COVID-19. The chance of mortality was two times higher in the patients with underlying diseases. Besides, one unit of increase in oxygen saturation could decrease the chance of mortality by 4%, which was significant. Considering the effect of drug regimen, the patients receiving favipiravir, remdesivir, Kaletra® had a lower chance of mortality (by 70, 30, and 50%) than those receiving IFN-β. However, these differences were not significant.
In terms of hospital stay, the present study results showed that COVID-19-related mortality was increased with a prolonged hospital stay. Moreover, the ICU-admitted patients had a significantly longer hospital stay. Considering the effect of the drug regimen, the patients receiving favipiravir and Kaletra® had a significantly shorter hospital stay than those receiving IFN-β. However, those receiving remdesivir had a longer hospital stay than patients receiving IFN-β, while the difference was not significant. Additionally, the patients admitted to ICU had significantly higher treatment costs. The variables of underlying disease, age, and gender had no significant effect on treatment costs.
4 Discussion
The COVID-19 pandemic was announced by the WHO in March 2020. This novel virus spread throughout the world very rapidly. COVID-19 is a respiratory infection affecting the overall health of the infected patients. This virus and the preventive measures taken for the related pandemic have challenged all interpersonal and social interactions by establishing several social-distancing and self-isolation measures (Singh 2020). Limited studies have investigated the costs and outcomes of COVID-19 drugs. A study on this topic, which was performed at the COVID-19 referral hospitals in the Fars province, Iran, showed that the pandemic imposed a heavy burden on the economies of the involved countries (Ghaffari Darab et al. 2021). Another study in South Africa reported that administration of remdesivir and dexamethasone for the patients without mechanical ventilation and those in need of mechanical ventilation, respectively, could lead to reduced costs due to shorter ICU stay than the standard care (Jo et al. 2021). Moreover, antiviral therapy, as an independent intervention or a part of a multifaceted strategy was mentioned to be cost-effective for managing respiratory infection outbreaks with a high chance of mortality, such as COVID-19 (Dawoud and Soliman 2020).
An objective of the present study was to investigate the relationship between demographics and COVID-19 severity with the related costs, reporting the significant effect of age and underlying diseases on the treatment outcomes (mortality and hospital stay). Our findings are compatible with those of the previous studies. The chance of mortality due to COVID-19 was reported to be higher in male patients and those with underlying diseases, including diabetes mellitus, hypertension, cardiovascular diseases, and renal disorders, compared to other patients (Sepandi et al. 2020). Moreover, a study in China on 541 patients with COVID-19, of which 26.6% had cardiovascular diseases, reported a mortality rate of 22.2% in those with cardiovascular diseases compared to a mortality rate of 9.8% in the total study participants. Besides, the patients with cardiovascular diseases were more likely to develop hepatic dysfunction and elevated levels of creatinine and lactate dehydrogenase (Zhang et al. 2020). Another study in China included 1590 patients with COVID-19 whereof 25.1% had at least one underlying disease. The most common underlying disease was hypertension (16.9%) followed by diabetes (8.2%), while 130 patients (8.2%) had two or more underlying diseases. The study reported that the patients with any underlying disease had poorer clinical outcomes than others (Guan et al. 2020). Additionally, a study on 203 patients with COVID-19 in Wuhan, China, reported that male gender, underlying diseases, higher duration between the onset of symptoms and hospitalization, renal dysfunction, and elevated procalcitonin levels had significant relationships with mortality (Chen et al. 2020). Therefore, it seems that older patients with underlying diseases should receive special care and be prioritized for more specialized treatments.
The present study intended to perform an analysis of the costs and outcomes of different drug regimens used for COVID-19 treatment. To achieve this goal, favipiravir, remdesivir, IFN-β, and Kaletra® were evaluated, because these four drugs were included in the COVID-19 treatment protocols of Iran at the time of this study. However, some other medications, such as dexamethasone, and monoclonal antibodies, including baricitinib, tofacitinib, tocilizumab, and sarilumab, were also added to the treatment protocols and could be prescribed based on the patients' conditions (https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/ accessed 4 January 2021). According to our findings, the patients taking favipiravir had the lowest chance of mortality and shortest hospital stay, while those receiving remdesivir presented the highest chance of mortality and longest hospital stay, which might be probably explained by the fact that most patients with severe COVID-19 and those who were ICU-admitted received remdesivir. Other studies in this regard have shown different findings. A randomized clinical trial by Spinner et al. reported that remdesivir led to clinical benefits in severe COVID-19 compared to placebo, while its effect was unknown in the patients with moderate COVID-19 (Spinner et al. 2020). Another study on the ICU-admitted patients with COVID-19 in Bangladesh showed that the patients receiving favipiravir had the longest hospital stay, while those receiving a combination of favipiravir, remdesivir, and convalescent plasma had the shortest hospital stay (Nasir 2021). On the other hand, no positive impact on mortality rates was reported upon remdesivir usage in two studies (Al-Abdouh 2021; Singh 2021). Besides, some concerns about its side effects are mentioned (Negahdaripour 2021).
In a double-blinded study on 1062 patients in two groups who were hospitalized due to COVID-19, Beigel et al. investigated the effectiveness of remdesivir compared to placebo. A group received remdesivir (200 mg on the first day and 100 mg daily on the next nine days) for 10 days, while the other group received the placebo. This study demonstrated the effect of remdesivir on reducing the hospital stay and pulmonary involvement of the patients (Beigel et al. 2020). Moreover, a clinical study investigated the effect of remdesivir by administering 200 mg remdesivir on the first day and 100 mg daily on the next nine days for hospitalized patients with COVID-19, reporting improvements in 68% of these patients (Grein et al. 2020). Besides, a study by Spinner et al. on 596 patients with moderate COVID-19 compared the effects of remdesivir for five and 10 days with the standard care, reporting no significant difference between remdesivir treatment for 5 and 10 days, while the 5-day remdesivir treatment course was significantly more beneficial than the standard care. Moreover, remdesivir treatment for five and 10 days could significantly reduce the recovery duration. The standard care in the mentioned study was supportive care, such as controlling the patients’ oxygen saturation (Spinner et al. 2020). However, a direct comparison of the results of different studies might not be meaningful due to variations in the clinical conditions of the study populations, so an accurate conclusion is difficult to make.
According to our results, the patients receiving favipiravir and Kaletra® had significantly shorter hospital stay than those receiving IFN-β. Moreover, favipiravir, remdesivir, and Kaletra® could reduce the chance of mortality by 70, 30, and 50% compared to IFN-β, respectively. A study by Cai et al. investigated the effects of favipiravir compared to lopinavir/ritonavir in patients with COVID-19. The study group included the patients receiving favipiravir and inhalatory IFN-α, while the control group received lopinavir/ritonavir and inhalatory IFN-α. The comparative indices included changes in the pulmonary CT scan, the time needed for viral clearance, and drug side effects. The study reported that the favipiravir group had a significantly faster viral clearance and improved CT scan compared to the control group. However, more side effects were reported in the favipiravir group (Cai et al. 2020).
In another study by Cao et al., the effect of Kaletra® was compared to standard care. The study population were 199 patients with COVID-19 who had respiratory problems and an oxygen saturation of 94% or lower. No positive effect was reported for this drug. The standard care in the mentioned study was supplemental oxygen, invasive or non-invasive ventilation, antibiotic treatment, blood pressure management, hemodialysis, and extracorporeal membrane oxygenation (Cao et al. 2020).
Moreover, patients receiving IFN-β had a higher chance of mortality than those receiving favipiravir and Kaletra®, which might be explained by the administration of IFN-β in the advanced stages of the disease that may exacerbate the patients' conditions (Bagheri et al. 2021). Considering the mechanism of action of IFN-β, it can only be administered in the initial stages of the disease, and it may aggravate the overreaction of the immune system and increase the inflammatory reactions if administered during the acute phase of the disease (Bagheri et al. 2021). The COVID-19 treatment protocols do not recommend IFN-β for treating the patients with severe or critical COVID-19, except for the clinical trials (AIII). However, there is insufficient evidence to recommend for or against IFN-β for the early (less than seven days after the onset of symptoms) treatment of mild to moderate COVID-19 (https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/interferons/ accessed 4 January 2022). IFN with or without arbidol was shown to reduce the viral load in the respiratory tract in a study. Moreover, it decreased the serum levels of inflammatory factors (Zhou et al. 2020). Additionally, the antiviral effects of IFN have been confirmed in several other in vitro and in vivo studies (Park and Iwasaki 2020).
Considering the COVID-19 pathogenesis and its interactions with the immune system mechanisms, especially down-regulation of IFNs, it seems that this drug is only beneficial if administered at the early stages of the disease (within 5–6 days after onset of the symptoms). However, at more advanced stages of the disease, the immune system might present an overreaction, leading to cytokine storm, which could end in pulmonary tissue destruction, and some other complications of the disease. Therefore, administration of IFN-β at this time is not useful and can exacerbate the patient's condition. Unfortunately, identifying the transition point between the early and inflammatory stages of COVID-19 is difficult using the patient's conditions or routine laboratory findings, considering that it could vary slightly in different patients (Nadimi Parashkouhi et al. 2021). Moreover, determining the exact onset of the disease is difficult, as when a COVID-19 patient is admitted to a hospital, several days may have already passed since the onset of symptoms. Such conditions could justify the controversial results of the previous studies on the effectiveness of IFN-β in COVID-19.
Furthermore, favipiravir showed the lowest chance of mortality in our study. A study by Yamamura et al. investigated the effectiveness of a combination of oral favipiravir, methylprednisolone, and unfractionated heparin in the hospitalized patients with COVID-19 who were not under mechanical ventilation. They reported that this combination could relatively manage the inflammatory manifestations of COVID-19 (Yamamura et al. 2020).
While there have been many controversies regarding the benefits of the mentioned drugs in the management of COVID-19 patients, recently, two other antiviral drugs have been recommended to treat patients with COVID-19, They include Paxlovid™ (nirmatrelvir in combination with ritonavir as booster) originated in Pfizer, and molnupiravir (Lagevrio®) introduced by MSD (Drożdżal et al. 2021; Mahase 2021; Hashemian et al. 2022). Paxlovid™, a SARS-CoV-2 protease inhibitor, can be administered orally at the first signs of infection or at the first knowledge of exposure and potentially helps to prevent severe illness that can lead to hospitalization and death (available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate. accessed 4 January 2022).
Molnupiravir is a nucleoside analog that interferes with the virus’ replication. It reduced the risk of hospital admission or death by around 50% in non-hospitalized adults who had mild to moderate COVID-19 but were at risk of severe disease (https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/ accessed 4 January 2022).
5 Conclusion
After nearly two years from the pandemic start, there are still unanswered questions regarding SARS-CoV-2 pathogenesis, its different outcomes in various populations and patients, and therapeutic approaches. Hence, findings about COVID-19 therapies could be parts of a puzzle leading together to a more complete picture of this disease in future.
The high expenses and prolonged hospital stay in the patients receiving remdesivir observed in this study suggest that this drug might not be a proper candidate for COVID-19 treatment compared to other antiviral drugs despite its routine administration in COVID-19 patients. On the other hand, favipiravir showed a lower chance of mortality and more cost-effectiveness than remdesivir in our analyses. However, considering the different conditions of the patients included in the present study, further investigations are needed to conclude more accurately. Moreover, investigating the co-effect of corticosteroids, as the efficacious therapeutic approach in severe COVID-19, in future research is recommended. Totally, different records are found in various studies on COVID-19 patients regarding the clinical benefits of indicated regimens, which are sometimes controversial. This might be due to very varied conditions of patients, diversities in administered protocols in different hospitals, and many other unknown reasons such as genetic variations, and so on. This study was performed before O-micron emergence. However, since remdesivir is still used, the obtained data could be helpful.
References
Al-Abdouh ABA, Barbarawi M, Jabri A, Kumar A, Fashanu OE et al (2021) Remdesivir for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials. Contemp Clin Trials 101:106272
Bagheri A, Moezzi SMI, Mosaddeghi P et al (2021) Interferon-inducer antivirals: potential candidates to combat COVID-19. Int Immunopharmacol 91:107245
Bartsch SM, Ferguson MC, McKinnell JA, O’Shea KJ, Wedlock PT (2020) The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff 39(6):927–935
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC et al (2020) Remdesivir for the treatment of Covid-19—final report. New Eng J Med 383(19):1813–1826
Cai Q, Yang M, Liu D, Chen J, Shu D et al (2020) Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering 6(10):1192–1198
Cao B, Wang Y, Wen D, Liu W, Wang J et al (2020) A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. New Eng J Med 382(19):1787–1799
Chen T, Dai Z, Mo P, Li X, Ma Z et al (2020) Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered. Retrosp Study J Gerontol: Series A 75(9):1788–1795
Dawoud DM, Soliman KY (2020) Cost-effectiveness of antiviral treatments for pandemics and outbreaks of respiratory illnesses, including COVID-19: a systematic review of published economic evaluations. Value Health 23(11):1409–1422
Doggrell SA (2020) Remdesivir, a remedy or a ripple in severe COVID-19? Expert Opin Investig Drugs 29:1195–1198
Drożdżal S, Rosik J, Lechowicz K, Machaj F, Szostak B, Przybyciński J et al (2021) An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist Updat 59:100794
Elshabrawy HA (2020) SARS-CoV-2: an update on potential antivirals in light of SARS-CoV antiviral drug discoveries. Vaccines 8:335
GhaffariDarab M, Keshavarz K, Sadeghi E, Shahmohamadi J, Kavosi Z (2021) The economic burden of coronavirus disease 2019 (COVID-19): evidence from Iran. BMC Health Serv Res 21(1):132
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E et al (2020) Compassionate use of remdesivir for patients with severe Covid-19. N Eng J Med 382(24):2327–2336
Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS et al (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Res J 55(5):2000547
Hashemian SMR, Pourhanifeh MH, Hamblin MR, Shahrzad MK, Mirzaei H (2022) Rd inhibitors and COVID-19: Is molnupiravir a good option? Biomed Pharmacother 146:112517
Jo Y, Jamieson L, Edoka I, Long L, Silal S et al (2021) Cost-effectiveness of remdesivir and dexamethasone for COVID-19 treatment in South Africa. Open Forum Inf Diss. https://doi.org/10.1093/ofid/ofab040
Li X, Geng M, Peng Y, Meng L, Lu S (2020) Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 10(2):102–108
Lotfi M, Rezaei N (2020) SARS-CoV-2: a comprehensive review from pathogenicity of the virus to clinical consequences. J Med Virol 92:1864–1874
Mahase E (2021) Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 375:n2713
Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC et al (2020) Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis 26:2005–2015
Molina JAD, Seow E, Heng BH, Chong WF, Ho B (2014) Outcomes of direct and indirect medical intensive care unit admissions from the emergency department of an acute care hospital: a retrospective cohort study. BMJ Open 4:e005553
Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN et al (2021) Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Resp Med 9:196–206
Mosaddeghi P, Shahabinezhad F, Dehghani Z, Farahmandnejad M, Taghipour MJ, Moghadami M, Nezafat N, Masoompour SM, Negahdaripour M (2021) Therapeutic approaches for COVID-19 based on the interferon-mediated immune responses. Curr Signal Transduct Ther 16:1
Nasir M, Perveen RA, Murshed M, Nazneen R, Talha KA (2021) Survival and biomarkers of COVID-19 patients treated with remdesivir and favipiravir in ICU during the peak of pandemic: a single center study in Bangladesh. JPRI 32(45):14–22
Negahdaripour M (2020) The rise and fall in therapeutic candidates for COVID-19. Iran J Med Sci 45:231–232
Negahdaripour M (2021) Post-COVID-19 hyperglycemia: a concern in selection of therapeutic regimens. Iran J Med Sci 46(4):235–236
Owji H, Negahdaripour M, Hajighahramani N (2020) Immunotherapeutic approaches to curtail COVID-19. Int Immunopharmacol 88:106924
Parashkouhi SN, Mosaddeghi P, Bagheri A, Moezzi SMI, Hoseini SMF et al (2021) The dual sides of interferon induction in COVID-19 treatment. Trend Pharm Sci 7(1):9–14
Park A, Iwasaki A (2020) Type I and Type III interferons–induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 27:870–878
Perlman S (2020) Another decade, another coronavirus. N Eng J Med 382:760–762
Sanchez-Felipe L, Vercruysse T, Sharma S, Ma J, Lemmens V et al (2020) A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature 590:320–325
Sepandi M, Taghdir M, Alimohamadi Y, Afrashteh S, Hosamirudsari H (2020) Factors associated with mortality in COVID-19 patients: a systematic review and meta-analysis. Iran J Public Health 49(7):1211–1221
Shen K, Yang Y, Wang T, Zhao D, Jiang Y et al (2020) Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. W J Pediatr 16(3):223–231
Singh J (2020) COVID-19 and its impact on society. Electron Res J Soc Sci. 2(I) Available at SSRN: https://ssrn.com/abstract=3567837
Singh SKD, Chugh A, Khera PS, Chugh VK (2021) Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis. BMJ Open 11:e048416
Spinner CD, Gottlieb RL, Criner GJ, ArribasLópez JR, Cattelan AM et al (2020) Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 324(11):1048–1057
Srinivas P, Sacha GL, Koval C (2020) Antivirals for COVID-19. Cleve Clin J Med. https://doi.org/10.3949/ccjm.87a.ccc030
V’Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19(3):155–170
Walmsley S, Bernstein B, King M, Arribas J, Beall G et al (2002) Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Eng J Med 346:2039–2046
Wise J (2020) Covid-19: remdesivir is recommended for authorisation by European Medicines Agency. BMJ 369:m2610
World Bank (2020) PPP conversion factor, GDP (LCU per international $) - Iran, Islamic Rep. Available at https://data.worldbank.org/indicator/PA.NUS.PPP?locations=IR, Accessed at 22 May 2020
Yamamura H, Matsuura H, Nakagawa J, Fukuoka H, Domi H et al (2020) Effect of favipiravir and an anti-inflammatory strategy for COVID-19. Crit Care 24(1):413
Zhang J, Lu S, Wang X, Jia X, Li J et al (2020) Do underlying cardiovascular diseases have any impact on hospitalised patients with COVID-19? Heart 106(15):1148
Zhou Q, Chen V, Shannon CP, Wei XS, Xiang X et al (2020) Interferon-α2b treatment for COVID-19. Front Immunol. https://doi.org/10.3389/fimmu.2020.01061
Funding
The authors wish to thank the Research Council of Shiraz University of Medical Sciences, Shiraz University of Medical Sciences in Iran for supporting the conduct of this research (Grant No. 22409).
Author information
Authors and Affiliations
Contributions
KHK and MN: contributed to the study conception and design. HB, KHK, and MN: were involved in data collection, statistical analysis, interpretation, manuscript drafting, and final review of the manuscript. MM, ESH, and IK: helped with the research idea, providing the data, and supervision of the research. JSH: helped in data collecting and data cleaning. MB: helped in statistical analysis and interpretation. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Behboodikhah, H., Shorafa, E., Karimzadeh, I. et al. Evaluation of the Costs and Outcomes of COVID-19 Therapeutic Regimens in Hospitalized Patients in Shiraz. Iran J Sci Technol Trans Sci 46, 1339–1347 (2022). https://doi.org/10.1007/s40995-022-01351-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-022-01351-0