Abstract
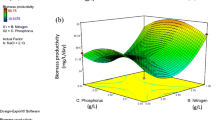
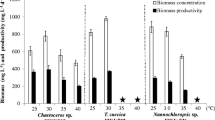
This study aimed to optimize growth medium composition for enhanced lipid and fatty acid production by the green halophilic chlorophyte Tetraselmis elliptica. The impact of different ratios of natural seawater, nitrogen source/concentration, carbon source/concentration, and trace metals on the growth, biomolecules, lipid accumulation, and lipid productivity was studied. In addition, fatty acid profile of the optimized growth medium was compared with that of the typical medium as a control, and biodiesel production as well as essential polyunsaturated fatty acids (PUFAs) were evaluated. Among all studied factors, 0.1 g L−1 KNO3, 0.01 g L−1 glycerol, and 7.0 mg L−1 FeSO4·7H2O significantly enhanced the lipid productivity of T. elliptica, and were used further to evaluate the impact of their combination. Cultivation of T. elliptica in the optimized medium showed enhancement of growth up to 16 days, which resulted in 30.9% and 17% increase in biomass and lipid productivities, respectively. Moreover, optimization of growth medium enhanced PUFAs proportion to 15.05% due to de novo synthesis of arachidonic acid and docosahexaenoic acids which represented 4.17% and 10.88%, respectively, of total fatty acids. Biodiesel characteristics of the optimized medium showed compliance of all studied parameters with the US standards. The present study provides new insights for commercial utilization of marine microalgae cultivated in optimized growth medium for dual purpose of ω-fatty acids and biodiesel production through biorefinery approach.
Graphical abstract









Similar content being viewed by others
References
Hill J, Nelson E, Tilman D et al (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci 103:11206–11210
Hoppe W, Bringezu S, Thonemann N (2016) Comparison of global warming potential between conventionally produced and CO2-based natural gas used in transport versus chemical production. J Clean Prod 121:231–237
Abomohra A, Faisal S, Ebaid R et al (2022) Recent advances in anaerobic digestion of lipid-rich waste: challenges and potential of seaweeds to mitigate the inhibitory effect. Chem Eng J 449:137829. https://doi.org/10.1016/J.CEJ.2022.137829
Von Zabeltitz C (1994) Effective use of renewable energies for greenhouse heating. Renew Energy 5:479–485. https://doi.org/10.1016/0960-1481(94)90419-7
Rittmann BE (2008) Opportunities for renewable bioenergy using microorganisms. Biotechnol Bioeng 100:203–212
Xiong W, Gao C, Yan D et al (2010) Double CO2 fixation in photosynthesis–fermentation model enhances algal lipid synthesis for biodiesel production. Bioresour Technol 101:2287–2293. https://doi.org/10.1016/J.BIORTECH.2009.11.041
Manzoor M, Hussain A, Ahmad Q-A et al (2022) Biodiesel quality assessment of microalgae cultivated mixotrophically on sugarcane bagasse. Sustain Energy Technol Assessments 53:102359. https://doi.org/10.1016/j.seta.2022.102359
Abomohra A, Jin W, Tu R et al (2016) Microalgal biomass production as a sustainable feedstock for biodiesel: current status and perspectives. Renew Sustain Energy Rev 64:596–606. https://doi.org/10.1016/j.rser.2016.06.056
Wang S, Mukhambet Y, Esakkimuthu S, Abomohra A (2022) Integrated microalgal biorefinery – routes, energy, economic and environmental perspectives. J Clean Prod 348:131245. https://doi.org/10.1016/J.JCLEPRO.2022.131245
Abomohra A, Wagner M, El-Sheekh M, Hanelt D (2013) Lipid and total fatty acid productivity in photoautotrophic fresh water microalgae: screening studies towards biodiesel production. J Appl Phycol 25:931–936. https://doi.org/10.1007/s10811-012-9917-y
Abomohra A, Eladel H, El-Esawi M et al (2018) Effect of lipid-free microalgal biomass and waste glycerol on growth and lipid production of Scenedesmus obliquus: innovative waste recycling for extraordinary lipid production. Bioresour Technol 249:992–999
Esakkimuthu S, Krishnamurthy V, Wang S et al (2020) Application of p-coumaric acid for extraordinary lipid production in Tetradesmus obliquus: a sustainable approach towards enhanced biodiesel production. Renew Energy 157:368–376
Touliabah HES, Almutairi AW (2021) Effect of phytohormones supplementation under nitrogen depletion on biomass and lipid production of nannochloropsis oceanica for integrated application in nutrition and biodiesel. Sustain 13:1–12. https://doi.org/10.3390/su13020592
Almutairi AW, El-Sayed AEKB, Reda MM (2021) Evaluation of high salinity adaptation for lipid bio-accumulation in the green microalga Chlorella vulgaris. Saudi J Biol Sci 28:3981–3988. https://doi.org/10.1016/j.sjbs.2021.04.007
Abomohra A, Shang H, El-Sheekh M, et al (2019) Night illumination using monochromatic light-emitting diodes for enhanced microalgal growth and biodiesel production. Bioresour Technol 288:121514. https://doi.org/10.1016/j.biortech.2019.121514
Almarashi JQM, El-Zohary SE, Ellabban MA, Abomohra AEF (2020) Enhancement of lipid production and energy recovery from the green microalga Chlorella vulgaris by inoculum pretreatment with low-dose cold atmospheric pressure plasma (CAPP). Energy Convers Manag 204:112314. https://doi.org/10.1016/j.enconman.2019.112314
Li L, Huang J, Almutairi AW et al (2021) (2021) Integrated approach for enhanced bio-oil recovery from disposed face masks through co-hydrothermal liquefaction with Spirulina platensis grown in wastewater. Biomass Convers Biorefinery 1:1–12. https://doi.org/10.1007/S13399-021-01891-2
Shao W, Ebaid R, Abomohra A, Shahen M (2018) Enhancement of Spirulina biomass production and cadmium biosorption using combined static magnetic field. Bioresour Technol 265:163–169. https://doi.org/10.1016/j.biortech.2018.06.009
Han SFS-F, Jin W, Tu R et al (2016) Optimization of aeration for biodiesel production by Scenedesmus obliquus grown in municipal wastewater. Bioprocess Biosyst Eng 39:1073–1079. https://doi.org/10.1007/s00449-016-1585-x
Jain P, Minhas AK, Shukla S et al (2022) Bioprospecting indigenous marine microalgae for polyunsaturated fatty acids under different media conditions. Front Bioeng Biotechnol 10:842797. https://doi.org/10.3389/FBIOE.2022.842797
Bagul VP, Annapure US (2021) Isolation and characterization of docosahexaenoic acid-producing novel strain Aurantiochytrium sp. ICTFD5: a sterol with vitamin D-cholecalciferol, and cellulase and lipase producing thraustochytrid. Bioresour Technol Reports 14:100688. https://doi.org/10.1016/J.BITEB.2021.100688
Ruiz-Rodriguez A, Reglero G, Ibañez E (2010) Recent trends in the advanced analysis of bioactive fatty acids. J Pharm Biomed Anal 51:305–326. https://doi.org/10.1016/J.JPBA.2009.05.012
Almutairi AW (2022) Evaluation of halophilic microalgae isolated from Rabigh Red Sea coastal area for biodiesel production: screening and biochemical studies. Saudi J Biol Sci 29:103339. https://doi.org/10.1016/j.sjbs.2022.103339
UTEX (2022) Algal culture media recipes. In: UTEX Cult. Collect. Algae. https://utex.org/pages/algal-culture-media. Accessed 7 Aug 2022
SAG (2008) Medium Recipe Version 10.2008. In: Cult. Collect. Algae. http://sagdb.uni-goettingen.de/culture_media/05 Seawater Medium.pdf. Accessed 26 May 2022
Almutairi AW (2022) Full utilization of marine microalgal hydrothermal liquefaction liquid products through a closed-loop route: towards enhanced bio-oil production and zero-waste approach. 3 Biotech 12:209. https://doi.org/10.1007/s13205-022-03262-8
Abomohra A, El-Sheekh M, Hanelt D (2017) Screening of marine microalgae isolated from the hypersaline Bardawil lagoon for biodiesel feedstock. Renew energy 101:1266–1272
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:495–509
Christie W (1993) Preparation of ester derivatives of fatty acids for chromatographic analysis. In: Adlof R (ed) Advances in lipid methodology – two. Oily Press, Dundee, pp 69–111
Abomohra A, Jin W, El-Sheekh M (2016) Enhancement of lipid extraction for improved biodiesel recovery from the biodiesel promising microalga Scenedesmus obliquus. Energy Convers Manag 108:23–29
Hoekman SK, Broch A, Robbins C et al (2012) Review of biodiesel composition, properties, and specifications. Renew Sustain Energy Rev 16:143–169
Song M, Pei H, Hu W, Ma G (2013) Evaluation of the potential of 10 microalgal strains for biodiesel production. Bioresour Technol 141:245–251
Ramos MJ, Fernández CM, Casas A et al (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
El-Sheekh M, Abomohra AE-FAEF, Hanelt D (2013) Optimization of biomass and fatty acid productivity of Scenedesmus obliquus as a promising microalga for biodiesel production. World J Microbiol Biotechnol 29:915–922. https://doi.org/10.1007/s11274-012-1248-2
Alsull M, Omar WMW (2012) Responses of Tetraselmis sp. and Nannochloropsis sp . isolated from Penang National Park Coastal Waters, Malaysia, to the combined influences of salinity, light and nitrogen limitation. In: In International Conference on Chemical, Ecology and Environmental Sciences (ICEES 2012)
Adarme-Vega TC, Thomas-Hall SR, Lim DKY, Schenk PM (2014) Effects of long chain fatty acid synthesis and associated gene expression in microalga Tetraselmis sp. Mar Drugs 12:3381–3398. https://doi.org/10.3390/MD12063381
El-Sheekh M, Abomohra A (2021) Handbook of algal biofuels : Aspects of cultivation, conversion, and biorefinery. Elsevier. https://doi.org/10.1016/C2020-0-00532-3
Ratanaporn L, Papone Thidarat PM (2014) Effect of nitrogen and carbon sources on growth and lipid production from mixotrophic growth of Chlorella sp. KKU-S2. Int J Biol Biomol Agric Food Biotechnol Eng 8:368–371
Kong W, Song H, Cao Y et al (2013) The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. African J Biotechnol 10:11620–11630. https://doi.org/10.4314/ajb.v10i55
Arumugam M, Agarwal A, Arya MC, Ahmed Z (2013) Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour Technol 131:246–249. https://doi.org/10.1016/J.BIORTECH.2012.12.159
Shi J, Podola B (2007) Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 195(19):417–423. https://doi.org/10.1007/S10811-006-9148-1
Wang S, Yerkebulan M, Abomohra AEF et al (2019) Microalgae harvest influences the energy recovery: a case study on chemical flocculation of Scenedesmus obliquus for biodiesel and crude bio-oil production. Bioresour Technol 286:121371
Aparna G (2015) A study of macronutrients in soils of different places around indore, MP, India. Res J Chem Sci 5:53–56
El-Ghany A (2014) Effect of different concentrations of sodium nitrate, sodium chloride, and ferrous sulphate on the growth and lipid content of Chlorella vulgaris. J Agric Technol 10:339–353
Ördög V, Stirk WA, Bálint P et al (2016) Effect of temperature and nitrogen concentration on lipid productivity and fatty acid composition in three Chlorella strains. Algal Res 16:141–149
Boyle NR, Morgan JA (2009) Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii. BMC Syst Biol 3:1–14. https://doi.org/10.1186/1752-0509-3-4/TABLES/7
Wei A, Zhang X, Wei D et al (2009) Effects of cassava starch hydrolysate on cell growth and lipid accumulation of the heterotrophic microalgae Chlorella protothecoides. J Ind Microbiol Biotechnol 36:1383–1383. https://doi.org/10.1007/S10295-009-0624-X
Dittamart D, Pumas C, Pekkoh J, Peerapornpisal Y (2014) Effects of organic carbon source and light-dark period on growth and lipid accumulation of Scenedesmus sp. AARL G022. Maejo Int J Sci Technol 8:198–206. https://doi.org/10.14456/MIJST.2014.28
Li M, Alotaibi MKH, Li L, Abomohra AE-F (2022) Enhanced waste glycerol recycling by yeast for efficient biodiesel production: towards waste biorefinery. Biomass Bioenerg 159:106410. https://doi.org/10.1016/J.BIOMBIOE.2022.106410
Kong WB, Yang H, Cao YT et al (2013) Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotrophic culture. Food Technol Biotechnol 51:62–69
Tawfik A, Ismail S, Elsayed M et al (2022) Sustainable microalgal biomass valorization to bioenergy: key challenges and future perspectives. Chemosphere 296:133812. https://doi.org/10.1016/J.CHEMOSPHERE.2022.133812
Al-Homaidan AA, Alabdullatif JA, Al-Hazzani AA et al (2015) Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J Biol Sci 22:795–800. https://doi.org/10.1016/j.sjbs.2015.06.010
Sasireka G, Muthuvelayudham R (2015) Effect of salinity and iron stressed on growth and lipid accumulation in skeletonema costatum for biodiesel production. Res J Chem Sci 5(5):69–72
Sun Y (2017) Huang Y (2017) Effect of trace elements on biomass, lipid productivity and fatty acid composition in Chlorella sorokiniana. Brazilian J Bot 404(40):871–881. https://doi.org/10.1007/S40415-017-0395-Y
Naito K, Matsui M, Imai I (2005) Ability of marine eukaryotic red tide microalgae to utilize insoluble iron. Harmful Algae 4:1021–1032. https://doi.org/10.1016/J.HAL.2005.02.003
Bothe H, Schmitz O, Yates MG, Newton WE (2010) Nitrogen fixation and hydrogen metabolism in Cyanobacteria. Microbiol Mol Biol Rev 74:529–551. https://doi.org/10.1128/MMBR.00033-10/ASSET/D25604DE-BD78-4306-836D-FDBC832AD533/ASSETS/GRAPHIC/ZMR9990922580010.JPEG
Paul A, Hauck M (2006) Effects of manganese on chlorophyll fluorescence in epiphytic cyano- and chlorolichens. Flora Morphol Distrib Funct Ecol Plants 201:451–460. https://doi.org/10.1016/j.flora.2005.09.001
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722. https://doi.org/10.1016/J.BIORTECH.2007.09.073
Chen M, Tang H, Ma H et al (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649–1655. https://doi.org/10.1016/J.BIORTECH.2010.09.062
Brenna JT, Diau GY (2007) The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot Essent Fat Acids 77:247–250. https://doi.org/10.1016/J.PLEFA.2007.10.016
Colombo J, Jill Shaddy D, Kerling EH et al (2017) Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot Essent Fatty Acids 121:52–56. https://doi.org/10.1016/J.PLEFA.2017.05.005
Shobana R, Vijayalakshmi S, Deepanraj B, Ranjitha J (2021) Biodiesel production from Capparis spinosa L seed oil using calcium oxide as a heterogeneous catalyst derived from oyster shell. Mater Today Proc. https://doi.org/10.1016/j.matpr.2021.07.215
GR Srinivasan V Shankar S Chandra Sekharan et al (2020) Influence of fatty acid composition on process optimization and characteristics assessment of biodiesel produced from waste animal fat. Energy Sources, Part A Recover Util Environ Eff. https://doi.org/10.1080/15567036.2020.1771477
ASTM D6751–08 (2008) Standard specification for biodiesel fuel blend stock (B100) for middle distillate fuels. West Conshohocken, PA: American Society for Testing and Materials
EN 14214 (2008) Automotive fuels D fatty acid methylesters (FAME) for diesel engines. Requirements and Test Methods. Eur Comm Stand EN 14214:2008
Bajpai D, Tyagi VK (2006) Biodiesel: source, production, composition, properties and its benefits. J Oleo Sci 55:487–502. https://doi.org/10.5650/JOS.55.487
Van Gerpen J (2005) Biodiesel processing and production. Fuel Process Technol 86:1097–1107. https://doi.org/10.1016/J.FUPROC.2004.11.005
Igbum OG, Leke L, Okoronkwo MU et al (2013) Evaluation of fuel properties from free fatty acid compositions of methyl esters obtained from four tropical virgin oils. Int J Appl Chem 9:37–49
Okiemen F, Omosigho H (2008) On the fuel properties of methyl esters of palm kernel oil. Niger J Appl Sci 26:90–94
Lapuerta M, Sanz-Argent J, Raine RR (2014) Ignition characteristics of diesel fuel in a constant volume bomb under diesel-like conditions. Effect of the Operation Parameters. Energy Fuels 28:5445–5454. https://doi.org/10.1021/EF500535J
Acknowledgements
The authors are grateful to the Egyptian Ministry of Higher Education & Scientific Research and National Natural Science Foundation of China for funding the present work.
Funding
This work was funded by the Egyptian Ministry of Higher Education & Scientific Research and National Natural Science Foundation of China (52050410328).
Author information
Authors and Affiliations
Contributions
R. S. Marey: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft. A. M. Abo-Shady: conceptualization, methodology, writing—review and editing, funding acquisition. H. M. Khairy: formal analysis, writing − review and editing. A. M. Abd El-Moneim: formal analysis, methodology. Abdelfatah Abomohra: conceptualization, data validation, writing—review and editing, funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marey, R.S., Abo-Shady, A.M., Khairy, H.M. et al. Enhanced lipid production and essential ω-fatty acids synthesis by the hypersaline biodiesel-promising microalga Tetraselmis elliptica through growth medium optimization. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03290-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03290-7