Abstract
Aims/hypothesis
Cancer has contributed to an increasing proportion of diabetes-related deaths, while lifestyle management is the cornerstone of both diabetes care and cancer prevention. We aimed to evaluate the associations of combined healthy lifestyles with total and site-specific cancer risks among individuals with diabetes.
Methods
We included 92,239 individuals with diabetes but without cancer at baseline from five population-based cohorts in the USA (National Health and Nutrition Examination Survey and National Institutes of Health [NIH]-AARP Diet and Health Study), the UK (UK Biobank study) and China (Dongfeng-Tongji cohort and Kailuan study). Healthy lifestyle scores (range 0–5) were constructed based on current nonsmoking, low-to-moderate alcohol drinking, adequate physical activity, healthy diet and optimal bodyweight. Cox regressions were used to calculate HRs for cancer morbidity and mortality, adjusting for sociodemographic, medical and diabetes-related factors.
Results
During 376,354 person-years of follow-up from UK Biobank and the two Chinese cohorts, 3229 incident cancer cases were documented, and 6682 cancer deaths were documented during 1,089,987 person-years of follow-up in the five cohorts. The pooled multivariable-adjusted HRs (95% CIs) comparing participants with 4–5 vs 0–1 healthy lifestyle factors were 0.73 (0.61, 0.88) for incident cancer and 0.55 (0.46, 0.67) for cancer mortality, and ranged between 0.41 and 0.63 for oesophagus, lung, liver, colorectum, breast and kidney cancers. Findings remained consistent across different cohorts and subgroups.
Conclusions/interpretation
This international cohort study found that adherence to combined healthy lifestyles was associated with lower risks of total cancer morbidity and mortality as well as several subtypes (oesophagus, lung, liver, colorectum, breast and kidney cancers) among individuals with diabetes.
Graphical abstract

Similar content being viewed by others

Introduction
Diabetes has posed an increasing threat to global public health, affecting 537 million adults and responsible for 6.7 million deaths and 966 billion US dollars in health expenditure in 2021 worldwide [1]. Traditional clinical management of diabetes has focused on the prevention of microvascular and macrovascular complications, and deaths caused by vascular diseases have significantly declined [2, 3]. Meanwhile, cancer has contributed to an increasing proportion of deaths among individuals with diabetes due to the neglect of cancer prevention during diabetes care, and cancer has outrun vascular disease as the leading cause of mortality associated with diabetes in the UK [2, 3]. Given the established associations between diabetes and increased risks of some cancers [4], more attention should be devoted to identifying modifiable cost-effective measures for cancer prevention in the clinical management of diabetes.
Lifestyle management is a fundamental aspect of both diabetes care and cancer prevention [5, 6]. However, evidence regarding the effects of combined lifestyles on cancer prevention among individuals with diabetes is limited. The Look Action for Health in Diabetes (Look AHEAD) randomised trial found no effects of intensive lifestyle interventions on cancer risks among adults with type 2 diabetes; however, the interventions only aimed at weight loss through reducing caloric intake and increasing physical activity among individuals with overweight and obesity, without considering other lifestyle factors (e.g., tobacco smoking and alcohol drinking) and individuals with normal weight, and the sample size was calculated to assess the effects on CVD instead of cancer [7]. Meanwhile, no cohort studies have investigated the association between combined lifestyle factors and incident cancer among individuals with diabetes; a recent meta-analysis reported results for cancer mortality but had limitations including small sample sizes (three studies with 11,565 participants and 646 cancer deaths) and insufficient control of confounding from sociodemographic or diabetes-related features [8]. Thus, we leveraged data from five population-based prospective cohorts to investigate the associations of combined healthy lifestyles with total and site-specific cancer morbidity and mortality among individuals with diabetes from the USA, the UK and China.
Methods
Study population
We included individuals with prevalent diabetes from five cohorts in the USA (National Health and Nutrition Examination Survey [NHANES] [9] and National Institutes of Health-AARP [NIH-AARP] Diet and Health Study [10]), the UK (UK Biobank [11]) and China (Dongfeng-Tongji cohort [DFTJ] [12] and Kailuan study [13]). All cohorts invited participants to complete questionnaire surveys, and cohorts except the NIH-AARP Diet and Health Study also invited participants to complete physical examinations and collections of blood samples. According to diagnostic criteria for diabetes from the ADA [14], 126,274 participants were identified to have diabetes through self-reported physician-diagnosed diabetes; use of hypoglycaemic agents; and glycaemic biomarkers including HbA1c, fasting plasma glucose (FPG) and plasma glucose level for a 2 h OGTT (electronic supplementary material [ESM] Methods). Participants with incomplete information on lifestyle factors, outcomes or major covariates (n=25,055) or prevalent cancer (n=8980) were excluded, leaving 92,239 participants in the cancer mortality analyses (ESM Fig. 1). Follow-up information for incident cancer was only available for 47,252 participants from the UK and two Chinese cohorts, and follow-up information for site-specific cancer morbidity or mortality was available for 86,183 participants from all cohorts except NHANES. All participants provided informed consent, and all cohorts were approved by institutional review boards. Detailed study designs and inclusion/exclusion criteria are described in the ESM Methods.
Construction of healthy lifestyle score
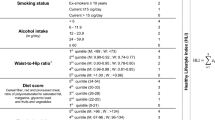
We constructed a healthy lifestyle score by summing the number of healthy lifestyle factors, i.e., current nonsmoking, low-to-moderate alcohol drinking, adequate physical activity, healthy diet, and optimal waist circumference or BMI [15], and the score ranged between 0 and 5, with higher values indicating healthier lifestyles. Detailed definitions of healthy levels of lifestyle factors are shown in Table 1. Briefly, current nonsmoking and consuming 1–28/14 g/day of alcohol for men/women were defined as healthy levels, respectively. Given different data collection tools across cohorts and different clinical cutoff values across populations, cohort-specific healthy levels of physical activity, diet and waist circumference/BMI were defined. The healthy levels of physical activity were defined as moderate-to-vigorous leisure-time physical activity of ≥150 min/week (NHANES 1999–2014) [16], top third of frequency of leisure-time physical activity (NHANES 1988–1994) [16], ≥20 min of physical activity ≥3 times/week (NIH-AARP Diet and Health Study) [17], top third of total physical activity (UK Biobank) [16], and ≥150 or ≥80 min/week of exercise (DFTJ or Kailuan study, respectively) [13, 18]. Dietary quality was assessed based on the Healthy Eating Index (two US cohorts) [16]; recent dietary recommendations for cardiovascular health (UK Biobank) [16]; intakes of fruits, vegetables and meat (DFTJ) [19]; and salt intake (Kailuan study) [13]. Considering the obesity paradox among individuals with diabetes, i.e. higher BMI was associated with a higher survival rate [20], we primarily used waist circumference to evaluate an individual’s obesity status [21], and the healthy levels were defined as <94/80 cm for men/women (NHANES and UK Biobank) according to the WHO recommendations and <90/85 cm for men/women (two Chinese cohorts) according to the Chinese Diabetes Society [22, 23]. BMI of 18.5–24.9 kg/m2 was defined as the healthy level in the NIH-AARP Diet and Health Study since only some of the participants reported waist circumference [24]. Detailed procedures for data collection are reported in the ESM Methods. Since few participants adopted 0 or 5 healthy lifestyle factors, those with 0–1 and 4–5 healthy lifestyle factors were merged to increase statistical power, respectively.
Follow-up time and outcomes
The follow-up time was calculated from the survey when participants first reported having diabetes, until the date of diagnosis of cancer, death or censoring date, whichever came first. The primary outcomes were total cancer incidence and mortality (except non-melanoma skin cancer), and the secondary outcomes were site-specific cancer morbidity or mortality, including bladder, breast, colorectum, oesophagus, kidney, liver, lung, pancreas, prostate and stomach cancers, and leukaemia; the numbers of other subtypes of cancers were too small (<200) and thus were not included in the analysis to avoid results of limited power. The data sources, censoring dates and International Classification of Diseases 9th or 10th Revision codes are detailed in the ESM Methods.
Statistical analysis
Given different study populations, study designs and data collection tools, analyses were conducted within each cohort first, and results were pooled using the random-effects model of meta-analysis. Such methods were widely used in previous pooling projects [25, 26]. HRs with 95% CIs of cancer morbidity and mortality comparing different healthy lifestyle score groups were estimated by Cox proportional hazards regression, which controlled for key baseline sociodemographic variables (i.e., age, sex, race, marital status, educational level, household income and employment status) and clinical factors (i.e., prevalent CVD and hypertension; family history of cancer, CVD and diabetes; use of antihypertensive, hypoglycaemic and lipid-lowering medications; years after diabetes diagnoses; FPG or HbA1c level; and total cholesterol level). These covariates were slightly varied in different cohorts due to data availability and different study populations, which are detailed in the ESM Methods.
Several subgroup analyses were conducted, and meta-regression was used to test the difference between subgroups by sociodemographic features (i.e., age [<65 vs ≥65 years] [27], sex and educational level [less than high school vs high school or higher]) and metabolic features (i.e. ideal BMI [yes vs no; defined as 18.5–24.9 kg/m2 in the USA and the UK and 18.5–23.9 kg/m2 in China] [22, 24], prevalent hypertension and prevalent dyslipidaemia [18]). We also conducted subgroup analyses by diabetes-related features, i.e. diabetes duration (new diagnoses through FPG or HbA1c screening vs self-reported diagnoses <5 years vs self-reported diagnoses ≥5 years), use of hypoglycaemic medications and achieving glycaemic target (yes vs no; HbA1c of <53 mmol/mol [7.0%] for those aged <65 years or <58 mmol/mol [7.5%] for those aged ≥65 years in the USA and the UK; or FPG of 4.4–7.2 or 5.0–7.2 mmol/l for those aged <65 or ≥65 years in China) [27, 28], in cohorts except the NIH-AARP Diet and Health Study.
To examine the contributions of different lifestyle factors, we first assessed the associations between five lifestyle factors and primary outcomes, with all lifestyle factors mutually adjusted for. Then, we reconstructed new healthy lifestyle scores by removing one lifestyle factor each time from the score, and five new scores with four factors were created. Participants were categorised into scores of 0–1, 2 and 3–4, and the removed factor was additionally adjusted for in the models.
Several sensitivity analyses were performed. First, we redefined the healthy level of alcohol drinking as none or low-to-moderate alcohol drinking (≤28/14 g/d for men/women) given the recent evidence indicating dose–response relationships between alcohol drinking and risks of multiple health outcomes [29]. Second, events occurring in the first 2 years were excluded to minimise the possibility of reverse causation. Third, to reduce possible confounding related to lifestyle change after the diagnosis of CVD, we excluded participants with prevalent CVD. Fourth, missing covariates were imputed by multiple imputations (five imputations; according to non-missing information) to reduce the impacts of non-responses [30].
Analyses within each cohort were conducted by SAS version 9.4 (SAS Institute, Cary, NC, USA), and meta-analyses were conducted by Stata version 14.0 (StataCorp, College Station, TX, USA). Two-sided p values <0.05 were considered statistically significant.
Results
Baseline characteristics of study participants
Of the 92,239 participants, 44,987 were from the USA (NHANES and NIH-AARP Diet and Health Study), 21,681 were from the UK (UK Biobank) and 25,571 were from China (DFTJ and Kailuan study). The mean baseline age ranged from 56.2 (Kailuan study) to 65.3 years (DFTJ) across cohorts (Table 2). The proportions of those with less than a high school degree were higher in the two Chinese cohorts (65.7–81.5%) than in the US and UK cohorts (27.0–31.9%). Current nonsmoking was less prevalent in the Kailuan study (65.0% vs 81.1–89.7% in other cohorts), which might be because the majority of the participants in the Kailuan study were male. Low-to-moderate alcohol drinking was more prevalent in the US and UK cohorts (34.2–76.0%) compared with the Chinese cohorts (14.5–22.5%), while optimal waist circumference/BMI was more prevalent in the Chinese cohorts (41.4–51.9%) compared with the US and UK cohorts (9.6–17.8%).
Baseline characteristics by lifestyle score groups in individual cohorts are shown in ESM Tables 1–5. Compared with those with 0–1 healthy lifestyle factors, participants with 4–5 healthy lifestyle factors were more likely to be older in the NIH-AARP Diet and Health Study, UK Biobank and Kailuan study; more likely to be male in the US and UK cohorts, but female in the Chinese cohorts; and less likely to have prevalent CVD (except for the NIH-AARP Diet and Health Study) and hypertension. Modest differences in some characteristics were observed between excluded and included participants (ESM Table 6).
Associations of healthy lifestyle score with cancer morbidity and mortality
In UK Biobank and the two Chinese cohorts, 3229 incident cancer cases were documented during 376,354 person-years of follow-up (mean = 8.0 years, Table 3). Compared with individuals with 0–1 healthy lifestyle factors, age-adjusted rates of incident cancer were lower among those with 4–5 healthy lifestyle factors, and HRs (95% CIs) comparing participants with 4–5 vs 0–1 healthy lifestyle factors were 0.83 (0.68, 1.00) in UK Biobank, 0.61 (0.45, 0.83) in the DFTJ, 0.70 (0.51, 0.97) in the Kailuan study and 0.73 (0.61, 0.88) after pooling the results from the three cohorts. As for site-specific cancers, we found that higher healthy lifestyle scores were associated with lower risks of oesophagus, lung, liver, colorectum, breast and kidney cancers, and the HRs comparing participants with 4–5 vs 0–1 healthy lifestyle factors ranged between 0.41 and 0.63; however, a one-point increase in the healthy lifestyle score was not associated with lower risks of breast (HR 0.92; 95% CI 0.82, 1.04) or kidney cancers (HR 0.91; 95% CI 0.80, 1.03) (Table 4). We found no statistically significant associations between the healthy lifestyle score and risks of bladder, pancreas, prostate or stomach cancers, or leukaemia. Given the unavailable data for incident cancer in the NIH-AARP Diet and Health Study, we investigated the associations between healthy lifestyle scores and incident site-specific cancers in UK Biobank and the two Chinese cohorts, and the results remained largely unchanged (ESM Table 7).
During 1,089,987 person-years of follow-up from the five cohorts (mean = 11.8 years), 6682 cancer deaths were documented in the five cohorts. HRs (95% CIs) comparing participants with 4–5 vs 0–1 healthy lifestyle factors were 0.37 (0.16, 0.85) in NHANES, 0.53 (0.48, 0.59) in the NIH-AARP Diet and Health Study, 0.67 (0.51, 0.86) in UK Biobank, 0.38 (0.24, 0.60) in the DFTJ, 0.73 (0.44, 1.22) in the Kailuan study and 0.55 (0.46, 0.67) after pooling the results from the five cohorts. The associations of healthy lifestyle scores with site-specific cancer mortality and morbidity were similar (ESM Table 7).
Subgroup analyses and sensitivity analyses
No statistically significant differences in the associations of the healthy lifestyle score with cancer morbidity and mortality were observed between subgroups by sociodemographic, metabolic and diabetes-related features (pbetween-group≥0.063, Fig. 1).
Associations of healthy lifestyle score with cancer morbidity and mortality in individuals with diabetes stratified by demographic, metabolic and diabetes-related features. The dots indicate the HRs comparing individuals with 4 or 5 vs 0 or 1 healthy lifestyle factors, and the horizontal lines indicate the 95% CIs. aData from the NIH-AARP Diet and Health Study were not included in the stratified analyses by diabetes-related features due to unavailable information. bThe number of cases was less than the total number. For incident cancer, BMI was not measured among 141 participants (eight had incident cancer). For cancer mortality, BMI was not measured among 188 participants (five died from cancer)
As for individual lifestyle factors, only current nonsmoking and low-to-moderate alcohol drinking were associated with HRs (95% CIs) of 0.64 (0.49, 0.82) and 0.86 (0.77, 0.95) for incident cancer, respectively, and current nonsmoking, low-to-moderate alcohol drinking and healthy diets were associated with HRs (95% CIs) of 0.53 (0.41, 0.68), 0.88 (0.78, 1.00) and 0.88 (0.84, 0.93) for cancer mortality, respectively (ESM Table 8). The associations were attenuated when tobacco smoking was removed from the healthy lifestyle score, and the HRs (95% CIs) comparing 3–4 vs 0–1 healthy lifestyle factors were 0.89 (0.79, 1.02) for cancer morbidity and 0.80 (0.75, 0.86) for cancer mortality (ESM Table 9). As for site-specific cancers, the healthy lifestyle score without tobacco smoking was only associated with breast and colorectum cancer risks, and the HRs (95% CIs) comparing 3–4 vs 0–1 healthy lifestyle factors were 0.64 (0.44, 0.92) and 0.73 (0.58, 0.91), respectively (ESM Table 10). Generally, when removing one lifestyle factor from the score each time, the associations of four-component lifestyle scores with risks of breast and kidney cancers were attenuated or even non-significant.
The associations remained largely consistent in sensitivity analyses redefining the healthy level of alcohol drinking, excluding participants who developed outcomes within the first 2 years of follow-up, excluding individuals with prevalent CVD or imputing missing covariates by multiple imputations (ESM Table 11).
Discussion
Combined healthy lifestyles were associated with significantly lower risks of total cancer morbidity and mortality among individuals with diabetes from the USA, the UK and China. We also observed associations of combined healthy lifestyles with lower risks of oesophagus, lung, liver, colorectum, breast and kidney cancers. The associations were consistent across cohorts and subpopulations with different sociodemographic, metabolic and diabetes-related features. However, the associations between the healthy lifestyle score and incident cancer were attenuated when tobacco smoking was removed from scores.
The disease burden related to cancer has reduced slowly among individuals with diabetes in recent decades, and cancer has even evolved into the leading cause of diabetes-related death in the UK [2, 3]. However, studies have seldom investigated the associations of combined healthy lifestyles, the cornerstone of diabetes care and cancer prevention, with cancer morbidity and mortality among individuals with diabetes. The Look AHEAD randomised clinical trial found that 4 years of intensive lifestyle intervention designed for weight loss could not reduce the incidence of total, obesity-related or non-obesity-related cancers compared with the diabetes support and education group during a median follow-up of 11 years [7]. However, several limitations of the study restricted the generalisability of the findings. First, the sample size was calculated to detect differences in major cardiovascular events between groups, and the analysis of cancer risks might be underpowered (4859 participants and 684 incident cancer cases) [31]. Second, the lifestyle intervention was not comprehensive: the study only considered weight loss through diet and exercise [7] and did not consider tobacco smoking and alcohol drinking which are established carcinogens [32]. Third, the trial was conducted among individuals with overweight/obesity, and the results might not apply to individuals with normal weight [7]. Thus, large population-based cohort studies are desperately needed to provide more solid evidence. To the best of our knowledge, this is the first cohort study to investigate the association between combined healthy lifestyles and incident cancer among individuals with diabetes, and highlighting the potential benefits of comprehensive lifestyle management for cancer prevention among individuals with diabetes. The result was consistent with previous studies from the general population (HR comparing individuals with the healthiest vs the least-healthy lifestyles was 0.71; 95% CI 0.66, 0.76) [15].
A recent meta-analysis of three cohort studies found that the healthiest lifestyles were associated with a 31% lower risk of cancer mortality among individuals with type 2 diabetes [8]. However, the sample size was small (646 cancer deaths), and the median follow-up duration was short (<8 years in two studies) [33,34,35]. Besides, none of them controlled for both sociodemographic and diabetes-related confounders [33,34,35]. Our study leveraged data from 92,239 participants with diabetes and documented 6682 cancer deaths during >1 million person-years of follow-up, and found the healthiest lifestyles were associated with a 45% lower risk of cancer mortality compared with the least-healthy lifestyles, which was much stronger than previous studies [8]. Of note, the association of combined healthy lifestyles with cancer mortality was stronger than that with cancer morbidity, which was consistent with previous studies from general populations [15]. This might be because healthy lifestyles were associated with lower risks of more aggressive cancers (such as colorectum and liver cancers) rather than less aggressive cancers (such as prostate cancer), and individuals with healthier lifestyles tended to receive earlier diagnoses and better treatments, which were related to better prognosis and could further reduce mortality [15].
Given the heterogeneous aetiologies for different cancer subtypes [15], it is necessary to investigate the associations between combined lifestyles and site-specific cancers among individuals with diabetes. It is reported that diabetes is associated with higher risks of liver, pancreas, endometrium, colorectum, breast and bladder cancers [4]. The associations might be partly explained by shared risk factors between diabetes and cancer, and diabetes-related hyperinsulinaemia, hyperglycaemia and inflammation could also increase the cancer risk [4]. We found that the healthiest lifestyles were associated with 37–59% lower risks of oesophagus, lung, liver, colorectum, breast and kidney cancers, and a previous meta-analysis among general populations also found healthy lifestyles were associated with reduced risks of bladder and endometrium cancer [15]. These results suggested the potential benefits of healthy lifestyles for counteracting the increased risks of diabetes-related cancer subtypes. Although both the meta-analysis among general populations [15] and our study found the highest healthy lifestyle score group was associated with lower breast and kidney cancer risks, our study reported non-significantly linear associations of healthy lifestyle scores with breast and kidney cancer risks, and the associations were attenuated or even became non-significant when removing one lifestyle factor from the score each time; thus, the results should be interpreted cautiously.
Previous studies have reported associations of current smoking with increased risks of both smoking-related and other cancers; especially, current smokers had several-fold-higher risks of lung, laryngeal, pharyngeal, upper digestive tract and oral cancers [36, 37]. Our analysis also found the associations of tobacco smoking with cancer morbidity and mortality were the strongest compared with other lifestyle factors. Current nonsmoking was the only factor associated with lower risks of cancer morbidity and mortality across all cohorts, while the associations of other lifestyle factors with risks of cancer morbidity and mortality were inconsistent across cohorts. Besides, excluding tobacco smoking from the lifestyle score attenuated the associations of lifestyle scores with cancer morbidity and mortality, which was also found in a previous meta-analysis among general populations [15]. The findings highlighted the priority of avoiding tobacco smoking in lifestyle recommendations for cancer prevention among individuals with diabetes, as this was a mutual risk factor for diabetes-related complications including cancer [38, 39].
To our knowledge, our study investigated the associations of combined lifestyles with incident cancer and site-specific cancers among individuals with diabetes for the first time. The prospective design, large sample size, long-term follow-ups, and standardised variable definition and analytical methods ensured the validity and reliability of our findings, and consistent findings across different cohorts with diverse characteristics and subgroups consolidate the generalisability of our findings. However, several limitations should be acknowledged. First, due to the observational nature, causal inference cannot be made, and misclassification bias introduced by self-reported lifestyle information and residual confounding induced by unmeasured confounders (e.g., access to cancer screening, use of different types of glucose-lowering medications) were inevitable. Second, the characteristics of participants included and excluded from the analysis due to missing information were different, which might cause selection bias; however, the results of multiple imputations and the main analyses were similar. Third, serious conditions could propel participants to adopt healthier lifestyles, and reverse causation is possible; however, the results of different subgroups by diabetes-related features were similar, and the results after excluding events occurring in the first 2 years remained unchanged. Fourth, subgroup analyses were not designed a priori and might be underpowered, and the numbers of certain site-specific cancer cases were small; thus, the results should be cautiously interpreted. Fifth, diabetes was defined through single-measured biomarkers or self-reports, and misclassification was possible. Additionally, types of diabetes could not be differentiated; however, most individuals should have type 2 diabetes since >92.5% of participants were diagnosed with diabetes after 30 years of age. Sixth, different cohorts used varied definitions of healthy lifestyle factors due to different data collection tools and country-specific lifestyle recommendations; thus, associations of healthy lifestyle scores with outcomes cannot be directly compared across cohorts. However, we defined healthy lifestyles according to local practice and previous studies, which could distinguish individuals with the healthiest lifestyles from those with the least-healthy lifestyles (i.e., participants with 4–5 vs 0–1 healthy lifestyle factors), and the similar HRs across cohorts highlighted the extrapolation of the results. Seventh, due to lack of data, the association between combined healthy lifestyles and incident cancer was only investigated in UK Biobank and the two Chinese cohorts, with evidence lacking from the US populations.
Our analyses in five cohorts from three countries found that adhering to healthy lifestyles was associated with lower risks of cancer morbidity and mortality among individuals with diabetes with different sociodemographic, metabolic and diabetes-related features. Adhering to healthy lifestyles was also associated with lower risks of oesophagus, lung, liver, colorectum, breast and kidney cancers. Our findings highlight the urgent need for multi-component lifestyle management among individuals with diabetes for cancer prevention, and avoiding tobacco smoking should be prioritised. Future research should focus on site-specific cancers and the effects of longitudinal lifestyle changes on cancer morbidity and mortality in individuals with diabetes.
Data availability
Reasonable requests to access the data from the Dongfeng-Tongji cohort and the Kailuan study used in this study may be sent to the corresponding authors. Data from the US NHANES are available at www.cdc.gov/nchs/nhis/index.htm. Data from the US NIH-AARP Diet and Health Study are available on application at https://dietandhealth.cancer.gov/. Data from UK Biobank are available on application at www.ukbiobank.ac.uk/register-apply.
Change history
05 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00125-022-05802-6
Abbreviations
- DFTJ:
-
Dongfeng-Tongji cohort
- FPG:
-
Fasting plasma glucose
- Look AHEAD:
-
Look Action for Health in Diabetes
- NHANES:
-
National Health and Nutrition Examination Survey
- NIH-AARP:
-
National Institutes of Health-AARP
References
IDF Diabetes Atlas (2021) Diabetes around the world | 2021. Available from https://diabetesatlas.org/idfawp/resource-files/2021/11/IDFDA10-global-fact-sheet.pdf. Accessed 16 November 2021
Gregg EW, Cheng YJ, Srinivasan M et al (2018) Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 391(10138):2430–2440. https://doi.org/10.1016/s0140-6736(18)30314-3
Pearson-Stuttard J, Bennett J, Cheng YJ et al (2021) Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol 9(3):165–173. https://doi.org/10.1016/s2213-8587(20)30431-9
Giovannucci E, Harlan DM, Archer MC et al (2010) Diabetes and cancer: a consensus report. Diabetes Care 33(7):1674–1685. https://doi.org/10.2337/dc10-0666
Davies MJ, D'Alessio DA, Fradkin J et al (2018) Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 61(12):2461–2498. https://doi.org/10.1007/s00125-018-4729-5
Clinton SK, Giovannucci EL, Hursting SD (2020) The World Cancer Research Fund/American Institute for Cancer Research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr 150(4):663–671. https://doi.org/10.1093/jn/nxz268
Look AHEAD Research Group, Yeh HC, Bantle JP et al (2020) Intensive Weight Loss Intervention and Cancer Risk in Adults with Type 2 Diabetes: Analysis of the Look AHEAD Randomized Clinical Trial. Obesity (Silver Spring) 28(9):1678–1686. https://doi.org/10.1002/oby.22936
Zhang Y, Pan XF, Chen J et al (2020) Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Diabetologia 63(1):21–33. https://doi.org/10.1007/s00125-019-04985-9
Centers for Disease Control and Prevention/National Center for Health Statistics (2017) About the National Health and Nutrition Examination Survey. Available from www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed 12 June 2021
Schatzkin A, Subar AF, Thompson FE et al (2001) Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 154(12):1119–1125. https://doi.org/10.1093/aje/154.12.1119
Sudlow C, Gallacher J, Allen N et al (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12(3):e1001779. https://doi.org/10.1371/journal.pmed.1001779
Wang F, Zhu J, Yao P et al (2013) Cohort Profile: the Dongfeng-Tongji cohort study of retired workers. Int J Epidemiol 42(3):731–740. https://doi.org/10.1093/ije/dys053
Zhang Q, Zhou Y, Gao X et al (2013) Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke 44(9):2451–2456. https://doi.org/10.1161/STROKEAHA.113.678839
American Diabetes Association (2020) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1):S14–S31. https://doi.org/10.2337/dc20-S002
Zhang YB, Pan XF, Chen J et al (2020) Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer 122(7):1085–1093. https://doi.org/10.1038/s41416-020-0741-x
Zhang YB, Chen C, Pan XF et al (2021) Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ 373:n604. https://doi.org/10.1136/bmj.n604
Pelser C, Arem H, Pfeiffer RM et al (2014) Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer 120(10):1540–1547. https://doi.org/10.1002/cncr.28573
Lloyd-Jones DM, Hong Y, Labarthe D et al (2010) Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 121(4):586–613. https://doi.org/10.1161/circulationaha.109.192703
Han X, Wei Y, Hu H et al (2020) Genetic risk, a healthy lifestyle, and type 2 diabetes: the Dongfeng-Tongji cohort study. J Clin Endocrinol Metab 105(4):1242–1250. https://doi.org/10.1210/clinem/dgz325
Carnethon MR, De Chavez PJ, Biggs ML et al (2012) Association of weight status with mortality in adults with incident diabetes. JAMA 308(6):581–590. https://doi.org/10.1001/jama.2012.9282
Dallongeville J, Bhatt DL, Steg PH et al (2012) Relation between body mass index, waist circumference, and cardiovascular outcomes in 19,579 diabetic patients with established vascular disease: the REACH Registry. Eur J Prev Cardiol 19(2):241–249. https://doi.org/10.1177/1741826710394305
Pan XF, Wang L, Pan A (2021) Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol 9(6):373–392. https://doi.org/10.1016/S2213-8587(21)00045-0
World Health Organization (2011) Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008. Available from https://apps.who.int/iris/handle/10665/44583. Accessed 6 June 2021
WHO Consultation on Obesity, World Health Organization (2000) Obesity: preventing and managing the global epidemic: report of a WHO consultation. Available from https://apps.who.int/iris/handle/10665/42330. Accessed 21 July 2021
Sotos-Prieto M, Bhupathiraju SN, Mattei J et al (2017) Association of changes in diet quality with total and cause-specific mortality. N Engl J Med 377(2):143–153. https://doi.org/10.1056/NEJMoa1613502
Yuan J, He Q, Nguyen LH et al (2021) Regular use of proton pump inhibitors and risk of type 2 diabetes: results from three prospective cohort studies. Gut 70(6):1070–1077. https://doi.org/10.1136/gutjnl-2020-322557
American Diabetes Association (2020) 12. Older adults: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1):S152–S162. https://doi.org/10.2337/dc20-S012
American Diabetes Association (2020) 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1):S66–S76. https://doi.org/10.2337/dc20-S006
GBD Alcohol Collaborators (2018) Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392(10152):1015–1035. https://doi.org/10.1016/S0140-6736(18)31310-2
Yuan Y (2011) Multiple imputation using SAS software. J Stat Softw 45:1–25. https://doi.org/10.18637/jss.v045.i06
The Look AHEAD Research Group (2003) Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 24(5):610–628. https://doi.org/10.1016/s0197-2456(03)00064-3
American Cancer Society (2019) Known and probable human carcinogens. Available from https://www.cancer.org/cancer/cancer-causes/general-info/known-and-probable-human-carcinogens.html. Accessed 16 November 2021
Bonaccio M, Di Castelnuovo A, Costanzo S et al (2019) Impact of combined healthy lifestyle factors on survival in an adult general population and in high-risk groups: prospective results from the Moli-sani Study. J Intern Med 286(2):207–220. https://doi.org/10.1111/joim.12907
Lin CC, Li CI, Liu CS et al (2012) Impact of lifestyle-related factors on all-cause and cause-specific mortality in patients with type 2 diabetes: the Taichung Diabetes Study. Diabetes Care 35(1):105–112. https://doi.org/10.2337/dc11-0930
Pan XF, Li Y, Franco OH, Yuan JM, Pan A, Koh WP (2020) Impact of Combined Lifestyle Factors on All-Cause and Cause-Specific Mortality and Life Expectancy in Chinese: The Singapore Chinese Health Study. J Gerontol A Biol Sci Med Sci 75(11):2193–2199. https://doi.org/10.1093/gerona/glz271
Gandini S, Botteri E, Iodice S et al (2008) Tobacco smoking and cancer: a meta-analysis. Int J Cancer 122(1):155–164. https://doi.org/10.1002/ijc.23033
Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA (2008) Smoking and smoking cessation in relation to mortality in women. JAMA 299(17):2037–2047. https://doi.org/10.1001/jama.299.17.2037
American Diabetes Association (2020) 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2020. Diabetes Care 43(Suppl 1):S48–S65. https://doi.org/10.2337/dc20-S005
Pan A, Wang Y, Talaei M, Hu FB (2015) Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation 132(19):1795–1804. https://doi.org/10.1161/circulationaha.115.017926
Liu G, Li Y, Hu Y et al (2018) Influence of lifestyle on incident cardiovascular disease and mortality in patients with diabetes mellitus. J Am Coll Cardiol 71(25):2867–2876. https://doi.org/10.1016/j.jacc.2018.04.027
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
Y-BZ, X-FP, QL, GL and AP contributed to study design. Y-BZ, X-FP, QL, Y-FZ, J-XC and XH carried out data analysis. Y-BZ, X-FP, QL and T-TG drafted the first version of the manuscript. All authors contributed to data interpretation and final approval of the version to be published, and critically reviewed and edited the manuscript. X-FP, Y-XW, LML, K-QG, KY, H-DY, DY, M-AH, X-MZ, L-GL, TW, S-LW, GL and AP contributed to acquisition of data. X-FP (NIH-AARP Diet and Health Study), S-LW (Kailuan study), GL (UK Biobank) and AP (Dongfeng-Tongji cohort and National Health and Nutrition Examination Survey) are the guarantors of this work and, as such, had full access to the corresponding data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The research was supported by the National Natural Science Foundation of China (81930124, 82021005 and 82073554), Fundamental Research Funds for the Central Universities (2021GCRC075 and 2021GCRC076), Hubei Province Science Fund for Distinguished Young Scholars (2021CFA048) and the China Postdoctoral Science Foundation (2021M691129). The study sponsors/funders were not involved in the design of the study; the collection, analysis and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the order of the last three authors was incorrect.
Supplementary information
ESM
(PDF 560 kb)
Rights and permissions
About this article
Cite this article
Zhang, YB., Pan, XF., Lu, Q. et al. Associations of combined healthy lifestyles with cancer morbidity and mortality among individuals with diabetes: results from five cohort studies in the USA, the UK and China. Diabetologia 65, 2044–2055 (2022). https://doi.org/10.1007/s00125-022-05754-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-022-05754-x