Abstract
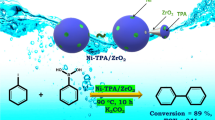
The present work consists of development of a novel heterogeneous catalyst comprising Keggin type polyoxometalate, 12-tungstophosphoric acid (PW12), and zeolite HY and its evaluation for the synthesis of alkyl levulinate. Various physicochemical methods were used for characterization, and the reaction optimization was studied by applying the impacts of the main reaction parameters (i.e. catalyst loading, molar ratio of acid to alcohol, time, and temperature). The catalyst shows 88% conversion and 99% selectivity towards n-butyl levulinate under optimized conditions such as 90°, 8 h with molar ratio of acid to alcohol (1:2) following second-order kinetics with activation energy of 80 kJ/mol. The catalyst is recovered and reused three times, always showing good performances. The catalyst was also found to be versatile and sustainable for a number of different bio-platform molecules, and alcohols with different chain lengths give % selectivity of respective esters between 49 and 100%. A comparison with the reported ones having phosphotungstate-based heterogeneous catalysts as well as classical ion-exchange resins shows superiority of the present catalyst in terms of lower amount (0.025 g) as well as highest TOF of 425 h−1.

















Similar content being viewed by others
Data availability
Supplementary information is available.
References
Al-Shaal MG, Ciptonugroho W, Holzhäuser FJ, Mensah JB, Hausoul PJC, Palkovits R (2015) Catalytic upgrading of α-angelica lactone to levulinic acid esters under mild conditions over heterogeneous catalysts. Catal Sci Technol 5:5168–5173. https://doi.org/10.1039/C5CY00446B
Luterbacher JS, Alonso DM, Dumesic JA (2014) Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chem 16:4816–4838. https://doi.org/10.1039/C4GC01160K
Neves P, Antunes MM, Russo PA, Abrantes JP, Lima S, Fernandes A, Pillinger M, Rocha SM, Riberio MF, Valente AA (2013) Production of biomass-derived furanic ethers and levulinate esters using heterogeneous acid catalysts. Green Chem 15:3367–3376. https://doi.org/10.1039/C3GC41908H
Krishnan V, McCalley JD (2016) The role of bio-renewables in national energy and transportation systems portfolio planning for low carbon economy. Renew Energy 91:207–223. https://doi.org/10.1016/j.renene.2016.01.052
Jahangiri H, Santos J, Hornung A, Ouadi M (2021) Thermochemical conversion of biomass and upgrading of bio-products to produce fuels and chemicals BT - catalysis for clean energy and environmental sustainability. In: Pant KK, Gupta SK, Ahmad E (eds) Biomass Conversion and Green Chemistry, Springer International Publishing, Cham, pp 1: 1–47. https://doi.org/10.1007/978-3-030-65017-9
Christensen E, Williams A, Paul S, Burton S, McCormick RL (2011) Properties and performance of levulinate esters as diesel blend components. Energy Fuels 25:5422–5428. https://doi.org/10.1021/ef201229j
Dharne S, Bokade VV (2011) Esterification of levulinic acid to n-butyl levulinate over heteropolyacid supported on acid-treated clay. J Nat Gas Chem 20:18–24. https://doi.org/10.1016/S1003-9953(10)60147-8
Maheria KC, Kozinski J, Dalai A (2013) Esterification of levulinic acid to n-butyl levulinate over various acidic zeolites. Catal Lett 143:1220–1225. https://doi.org/10.1007/s10562-013-1041-3
Nandiwale KY, Bokade VV (2014) Esterification of renewable levulinic acid to n-butyl levulinate over modified H-ZSM-5. Chem Eng Technol 38:246–252. https://doi.org/10.1002/ceat.201400326
Cirujano FG, Corma A, iXamena FXL (2015) Conversion of levulinic acid into chemicals: synthesis of biomass derived levulinate esters over Zr-containing MOFs. Chem Eng Sci 124:52–60. https://doi.org/10.1016/j.ces.2014.09.047
Peixoto AF, Soliman MMA, Pinto TV, Silva SM, Costa P, Alegria ECBA, Freire C (2021) Highly active organosulfonic aryl-silica nanoparticles as efficient catalysts for biomass derived biodiesel and fuel additives. Biomass Bioenergy 145:105936. https://doi.org/10.1016/j.biombioe.2020.105936
Yang F, Tang J (2019) Catalytic upgrading of renewable levulinic acid to levulinate esters using perchloric acid decorated nanoporous silica gels. ChemistrySelect 4:1403–1409. https://doi.org/10.1002/slct.201803608
Ramli NAS, Hisham NI, Amin NAS (2018) Esterification of levulinic acid to levulinate esters in the presence of sulfated silica catalyst. Sains Malays 47:1131–1138. https://doi.org/10.17576/jsm-2018-4706-08
Chermahini AN, Nazeri M (2017) Esterification of the levulinic acid with n-butyl and isobutyl alcohols over aluminum-containing MCM-41. Fuel Process Technol 167:442–450. https://doi.org/10.1016/j.fuproc.2017.07.034
Pachamuthua MP, Srinivasanb VV, Karvembub R, Luquec R (2019) Preparation of mesoporous stannosilicates SnTUD-1 and catalytic activity in levulinic acid esterification. Microporous Mesoporous Mater 287:159–166. https://doi.org/10.1016/j.micromeso.2019.05.061
Miao Z, Li Z, Zhao J, Si W, Zhou J, Zhuo S (2018) MoO3 supported on ordered mesoporous zirconium oxophosphate: an efficient and reusability solid acid catalyst for alkylation and esterification. Mol Catal 444:10–21. https://doi.org/10.1016/j.mcat.2017.10.028
Tejero MA, Ramírez E, Fité C, Tejero J, Cunill F (2016) Esterification of levulinic acid with butanol over ion exchange resins. Appl Catal A Gen 517:56–66. https://doi.org/10.1016/j.apcata.2016.02.032
Trombettoni V, Bianchi L, Zupanic A, Porciello A, Cuomo M, Piermatti O, Marrocchi A, Vaccaro L (2017) Efficient catalytic upgrading of levulinic acid into alkyl levulinates by resin-supported acids and flow reactors. Catalysts 7:235. https://doi.org/10.3390/catal7080235
Nakhate AV, Yadav GD (2016) Synthesis and characterization of sulfonated carbon based graphene oxide monolith by solvothermal carbonization for esterification and unsymmetrical ether formation. ACS Sustain Chem Eng 4:1963–1973. https://doi.org/10.1021/acssuschemeng.5b01205
Zhou S, Jiang D, Liu X, Chena Y, Yin D (2018) Titanate nanotubes-bonded organosulfonic acid as solid acid catalyst for synthesis of butyl levulinate. RSC Adv 8:3657–3662. https://doi.org/10.1039/C7RA12994G
Mao FF, Zhao W, Tao DJ, Liu X (2020) Highly efficient conversion of renewable levulinic acid to n-butyl levulinate catalyzed by sulfonated magnetic titanium dioxide nanotubes. Catal Lett 150:2709–2715. https://doi.org/10.1007/s10562-020-03177-0
Emrah Altuntepe E, Emel’yanenko VN, Forster-Rotgers M, Sadowski G, Verevkin SP, Held C (2017) Thermodynamics of enzyme-catalyzed esterifications: II. Levulinic acid esterification with short-chain alcohols. Appl Microbiol Biotechnol 101:7509–7521. https://doi.org/10.1007/s00253-017-8481-4
Zhoua L, Hea Y, Maa L, Jianga Y, Huanga Z, Yina L, Gao J (2018) Conversion of levulinic acid into alkyl levulinates: using lipase immobilized on meso-molding three-dimensional macroporous organosilica as catalyst. Bioresour Technol 247:568–575. https://doi.org/10.1016/j.biortech.2017.08.134
Badgujar KC, Bhanage BM (2015) Thermo-chemical energy assessment for production of energy-rich fuel additive compounds by using levulinic acid and immobilized lipase. Fuel Process Technol 138:139–146. https://doi.org/10.1016/j.fuproc.2015.05.015
Tian Y, Zhang R, Zhao W, Wen S, Xiang Y, Liu X (2020) A new sulfonic acid-functionalized organic polymer catalyst for the synthesis of biomass-derived alkyl levulinates. Catal Lett 150:3553–3560. https://doi.org/10.1007/s10562-020-03253-5
Wang H, Lu Y, Liu H, Yin Y, Liang J (2020) Preparation and application of magnetic nano-solid acid catalyst Fe3O4-PDA-SO3H. Energies 13:1484. https://doi.org/10.3390/en13061484
Zhou X, Li ZX, Zhang C, Gao XP, Dai YZ, Wang GY (2016) Efficient conversion of renewable levulinic acid to n-butyl levulinate catalyzed by ammonium and silver co-doped phosphotungstic acid. J Mol Catal A Chem 417:71–75. https://doi.org/10.1016/j.molcata.2016.03.006
Manikandan K, Cheralathan KK (2017) Heteropoly acid supported on silicalite−1 possesing intracrystalline nanovoids prepared using biomass − an efficient and recyclable catalyst for esterification of levulinic acid. Appl Catal A Gen 547:237–247. https://doi.org/10.1016/j.apcata.2017.09.007
Escobar AM, Blanco MN, Martínez JJ, Cubillos JA, Romanelli GP, Pizzio LR (2019) Biomass derivative valorization using nano core-shell magnetic materials based on Keggin-heteropolyacids: levulinic acid esterification kinetic study with N-butanol. J Nanomater 2019:5710708. https://doi.org/10.1155/2019/5710708
Pithadia D, Patel A, Hatiya V (2022) 2-Tungstophosphoric acid anchored to MCM-22, as a novel sustainable catalyst for the synthesis of potential biodiesel blend, levulinate ester. Renew Energy 187:933–943. https://doi.org/10.1016/j.renene.2022.01.106
Chena Y, Zhanga X, Dong M, Wua Y, Zhengc G, Huanga J, Guana X, Zheng X (2016) MCM-41 immobilized 12-silicotungstic acid mesoporous materials: structural and catalytic properties for esterification of levulinic acid and oleic acid. J Taiwan Inst Chem Eng 61:147–155. https://doi.org/10.1016/j.jtice.2015.12.005
Luana Q, Liua L, Gonga S, Lua J, Wang X, Lv D (2018) Clean and efficient conversion of renewable levulinic acid to levulinate esters catalyzed by an organic-salt of H4SiW12O40. Pross Saf Environ Prot 117:341–349. https://doi.org/10.1016/j.psep.2018.05.015
Misono M (1993) Catalytic chemistry of solid polyoxometalates and their industrial applications. Mol Eng 3:193–203. https://doi.org/10.1007/BF00999633
Cejka J, Centi G, Parientec JP, Roth WJ (2012) Zeolite-based materials for novel catalytic applications: opportunities, perspectives and open problems. Catal Today 179:2–15. https://doi.org/10.1016/j.cattod.2011.10.006
Lanzafame P, Barbera K, Papanikolaou G, Perathoner S, Centi G, Migliori M, Catizzone E, Giordano G (2017) Comparison of H+ and NH4+ forms of zeolites as acid catalysts for HMF etherification. Catal Today 304:97–102. https://doi.org/10.1016/j.cattod.2017.08.004
Li G, Pidko EA (2019) The nature and catalytic function of cation sites in zeolites: a computational perspective. ChemCatChem 11:134–156. https://doi.org/10.1002/cctc.201801493
Liu Z, Shi C, Wu D, He S, Ren B (2016) A simple method of preparation of high silica zeolite Y an its performance in the catalytic cracking of cumene. J Nanotechnol 2016:1486107. https://doi.org/10.1155/2016/1486107
Sahu HR, Rao GR (2000) Characterization of combustion synthesized zirconia powder by UV- vis, IR and other techniques. Bull Mater Sci 23:349–354. https://doi.org/10.1007/BF02708383
Ferreiraa P, Fonseca IM, Ramos AM, Vital J, Castanheiro JE (2010) Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Appl Catal B 98:94–99. https://doi.org/10.1016/j.apcatb.2010.05.018
Patel A, Pithadia D (2020) Low temperature synthesis of bio-fuel additives via valorisation of glycerol with benzaldehyde as well as furfural over a novel sustainable catalyst, 12-tungstosilicic acid anchored to ordered cubic nano-porous MCM-48. Appl Catal A Gen 602:117729. https://doi.org/10.1016/j.apcata.2020.117729
Ramli NAS, Amin NAS (2014) Fe/HY zeolite as an effective catalyst for levulinic acid production from glucose: characterization and catalytic performance. Appl Catal B 163:487–498. https://doi.org/10.1016/j.apcatb.2014.08.031
Deltcheff CR, Fournier M, Franck R, Thouvenot R (1983) Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(V1) and tungsten(V1) compounds related to the Keggin structure. Inorg Chem 22:207–216. https://doi.org/10.1021/IC00144A006
Ayad Z, Hussein HQ, Tabbakh BAA (2020) Synthesis and characterization of high silica HY zeolite by basicity reduction. AIP Conf Proc 2213:020168. https://doi.org/10.1063/5.0000278
dos Santos de Castro PR, Maia AAB, Angélica RS (2019) Study of the thermal stability of faujasite zeolite synthesized from Kaolin waste from the Amazon. Mater Res 22:e20190321. https://doi.org/10.1590/1980-5373-MR-2019-0321
Highfield JG, Moffat JB (1984) Characterization of 12-tungstophosphoric acid and related salts using photoacoustic spectroscopy in the infrared region II. Interactions with Pyridine. J Catal 89:185–195. https://doi.org/10.1016/0021-9517(84)90296-3
Freitas EF, Araújo AAL, Paiva MF, Dias SCL, Dias JA (2018) Comparative acidity of BEA and Y zeolite composites with 12- tungstophosphoric and 12-tungstosilicic acids. Mol Catal 458:152–160. https://doi.org/10.1016/j.mcat.2018.03.005
Okuhara T, Mizuno N, Misono M (1996) Catalytic chemistry of heteropoly compounds. Adv Catal 41:113–252. https://doi.org/10.1016/S0360-0564(08)60041-3
Mizunon N, Misono (1987) M pore structure and surface area of CsxH3-xPM12O40 (x=0-3, M=W, Mo). Chem Lett 16:967–970. https://doi.org/10.1246/cl.1987.967
Badgujara KC, Badgujara VC, Bhanage BM (2020) A review on catalytic synthesis of energy rich fuel additive levulinate compounds from biomass derived levulinic acid. Fuel Process Technol 197:106213. https://doi.org/10.1016/j.fuproc.2019.106213
Schüth F, Ward MD, Buriak JM (2018) Common pitfalls of catalysis manuscripts submitted to chemistry of materials. Chem Mater 30:3599–3600. https://doi.org/10.1021/acs.chemmater.8b01831
Puri BR, Sharma LR, Pathania MS (1962) Principles of physical chemistry. Vishal Publishing Co., p 1033–1111
Bond GC (1974) Heterogeneous catalysis: principles and applications. Clarendon Press, Oxford, p 4
Liang J, Liang Z, Zou R, Zhao Y (2017) Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks. Adv Mater 29:1701139. https://doi.org/10.1002/adma.201701139
Przypis M, Matuszek K, Chrobok A, Swadźba-Kwaśny M, Gillner D (2020) Inexpensive and tuneable protic ionic liquids based on sulfuric acid for the biphasic synthesis of alkyl levulinates. J Mol Liq 308:113166–113173. https://doi.org/10.1016/j.molliq.2020.113166
Mortazavi M, Chermahini AN, Mohammadbagheri Z (2019) Synthesis of hexyl levulinate as a potential fuel additive from levulinic acid over a solid acid catalyst. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2019.103420
Gupta SSR, Kantam ML (2019) Catalytic conversion of furfuryl alcohol or levulinic acid into alkyl levulinates using a sulfonic acid-functionalized hafnium-based MOF. Catal Commun 124:62–66. https://doi.org/10.1016/J.CATCOM.2019.03.003
Alamdari FR, Niri MN, Hazarkhania H (2018) A novel hydrogen-bonded silica-supported acidic ionic liquid: an efficient, recyclable and selective heterogeneous catalyst for the synthesis of diesters. J Chem Sci 130:48. https://doi.org/10.1007/s12039-018-1454-z
Lown AL, Peereboom L, Mueller SA, Anderson JE, Miller DJ, Lira CT (2014) Cold flow properties for blends of biofuels with diesel and jet fuels. Fuel 117:544–551. https://doi.org/10.1016/j.fuel.2013.09.067
Jing C, Jinhua L, Zhongxie D, Yuehua W, Zhen L, Min J, Xiaoqian R (2018) Lewis acid sites of Mg2+-modified polystyrene sulfonic acid resin catalysys for synthesis of dibutyl succinate. Quím Nova 41:613–618. https://doi.org/10.21577/0100-4042.20170230
Ramli NAS, Zaharudin NH, Amin NAS (2017) Esterification of renewable levulinic acid to levulinate esters using amberlyst-15 as a solid acid catalyst. J Teknol 79:137–142. https://doi.org/10.11113/jt.v79.8095
Lutz W (2014) Zeolite Y: synthesis, modification, and properties—a case revisited. Adv Mater Sci Eng 2014:724248. https://doi.org/10.1155/2014/724248
Acknowledgements
AP and MJ are thankful to the Department of Chemistry, The Maharaja Sayajirao University of Baroda for the infrastructural facilities and DST-FIST for BET surface area analysis. MJ is thankful to SHODH (ScHeme Of Developing High quality research, KCG/SHODH/2020-21/) for providing financial support.
Author information
Authors and Affiliations
Contributions
Anjali Patel, conceptualization, supervision, and writing — review and editing; Margi Joshi, formal analysis and investigation, methodology, and writing — original draft preparation; Shivani Sharma, preliminary experiments and analysis.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
No conflicts, informed consent, or human or animal rights are applicable to this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, A., Joshi, M. & Sharma, S. Designing of a novel heterogeneous catalyst comprising 12-tungstophosphoric acid and zeolite HY for the synthesis of bio-based esters. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03279-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03279-2