Abstract
The lignocellulosic residue cassava peel is an unexplored source of bioactive compounds, such as hemicellulose-based xylooligosaccharides (XOS) that present prebiotic properties. In this sense, this work aimed to produce XOS from cassava peels that were pretreated with NaOH (xylan extraction and lignin removal) followed by enzymatic hydrolysis (endo-1–4-β-xylanase). The cassava peels were pretreated sequentially: starch removal, alkaline hydrolysis, and enzymatic hydrolysis. The aqueous-mechanical reduction and sieving for 15 cycles removed ≈16% of starch (iodometric method). The alkaline pretreatments were carried out with 2, 4, and 6% (w/v) NaOH, 1:100/solid:liquid at 121 °C and 1.1 bar for 30 or 60 min. The enzymatic kinetics was evaluated with enzyme concentration at 0.5, 1.5, and 3.0% (v/v). The highest alkaline hydrolysis reached 34.20% of lignin removal (2% (w/v) NaOH for 30 min). The highest XOS yield was 396.5 mg XOS/g xylan after 48 h and 3.0% enzyme concentration. Regarding the mass balance, from 300 g of cassava peels (an agroindustrial residue), it is possible to obtain up to 3.27% XOS. Therefore, the fractionation of hemicellulose from cassava peels was technically viable from the concepts of biorefinery and bioeconomy, being one of the first researches to approach the extraction of xylan from cassava peel to obtain XOS through an enzymatic route with a highly promising yield.
Highlights
• The high starch content is a drawback to producing cassava peel-based xylooligosaccharides.
• Delignification and extraction of xylan from cassava peels by alkaline hydrolysis were performed.
• Xylooligosaccharides were produced by enzymatic hydrolysis with endo-1–4-β-xylanase.
• The highest yield of cassava peel-based xylooligosaccharides, already reported.
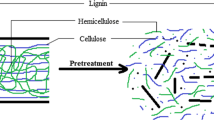
Graphical abstract






Similar content being viewed by others
Data availability
Not applicable.
References
Blagbrough IS, Bayoumi SAL, Rowan MG, Beeching JR (2010) Cassava: an appraisal of its phytochemistry and its biotechnological prospects. Phytochemistry 71:1940–1951. https://doi.org/10.1016/j.phytochem.2010.09.001
Mombo S, Dumat C, Shahid M, Schreck E (2017) A socio-scientific analysis of the environmental and health benefits as well as potential risks of cassava production and consumption. Environ Sci Pollut Res 24:5207–5221. https://doi.org/10.1007/s11356-016-8190-z
Otekunrin A, O, Sawicka B, (2019) Cassava, a 21st century staple crop: how can Nigeria harness its enormous trade potentials? Acta Sci Agric 3:194–202. https://doi.org/10.31080/asag.2019.03.0586
Adekunle A, Orsat V, Raghavan V (2016) Lignocellulosic bioethanol: a review and design conceptualization study of production from cassava peels. Renew Sustain Energy Rev 64:518–530. https://doi.org/10.1016/j.rser.2016.06.064
Sivamani S, Pandian A, Muthusamy C et al (2018) Evaluation of the potential of cassava-based residues for biofuels production. Rev Environ Sci Bio/Technology 17:553–570. https://doi.org/10.1007/s11157-018-9475-0
Cruz IA, Santos Andrade LR, Bharagava RN et al (2021) Valorization of cassava residues for biogas production in Brazil based on the circular economy: an updated and comprehensive review. Clean Eng Technol 4:100196. https://doi.org/10.1016/j.clet.2021.100196
Kayiwa R, Kasedde H, Lubwama M, Kirabira JB (2021) The potential for commercial scale production and application of activated carbon from cassava peels in Africa: a review. Bioresour Technol Reports 15:100772. https://doi.org/10.1016/j.biteb.2021.100772
Ndongo GK, Nsami NJ, Mbadcam KJ (2020) Ferromagnetic activated carbon from cassava (Manihot dulcis) peels activated by iron(III) chloride: synthesis and characterization. BioResources 15:2133–2146. https://doi.org/10.15376/biores.15.2.2133-2146
Babayemi OJ, O.J. Ifut UAI and LJI, (2010) Quality and chemical composition of cassava wastes ensiled with Albizia saman pods. Agric J 5:225–228. https://doi.org/10.3923/aj.2010.225.228
Pooja NS, Padmaja G (2015) Enhancing the enzymatic saccharification of agricultural and processing residues of cassava through pretreatment techniques. Waste Biomass Valori 6:303–315. https://doi.org/10.1007/s12649-015-9345-8
Versino F, García MA (2019) Particle size distribution effect on cassava starch and cassava bagasse biocomposites. ACS Sustain Chem Eng 7:1052–1060. https://doi.org/10.1021/acssuschemeng.8b04700
Sudaryanto Y, Hartono SB, Irawaty W et al (2006) High surface area activated carbon prepared from cassava peel by chemical activation. Bioresour Technol 97:734–739. https://doi.org/10.1016/j.biortech.2005.04.029
Murata Y, Nwuche CO, Nweze JE et al (2021) Potentials of multi-stress tolerant yeasts, Saccharomyces cerevisiae and Pichia kudriavzevii for fuel ethanol production from industrial cassava wastes. Process Biochem 111:305–314. https://doi.org/10.1016/j.procbio.2021.11.014
Olaoye RA, Afolayan OD, Adeyemi KA et al (2020) Adsorption of selected metals from cassava processing wastewater using cow-bone ash. Sci African 10:e00653. https://doi.org/10.1016/j.sciaf.2020.e00653
Aruwajoye GS, Sewsynker-Sukai Y, Kana EBG (2020) Valorisation of cassava peels through simultaneous saccharification and ethanol production: effect of prehydrolysis time, kinetic assessment and preliminary scale up. Fuel 278. https://doi.org/10.1016/j.fuel.2020.118351
Zúñiga-Arias D, Charpentier-Alfaro C, Méndez-Arias J, Rodríguez-Mora K (2022) Changes in the structure and composition of pineapple leaf fiber after alkali and ionic surfactant pretreatments and their impact on enzymatic hydrolysis. Prep Biochem Biotechnol 0:1–10. https://doi.org/10.1080/10826068.2021.2021233
Ma R, Bai Y, Huang H et al (2017) Utility of thermostable xylanases of Mycothermus thermophilus in generating prebiotic xylooligosaccharides. J Agric Food Chem 65:1139–1145. https://doi.org/10.1021/acs.jafc.6b05183
Jagtap S, Deshmukh RA, Menon S, Das S (2017) Xylooligosaccharides production by crude microbial enzymes from agricultural waste without prior treatment and their potential application as nutraceuticals. Bioresour Technol 245:283–288. https://doi.org/10.1016/j.biortech.2017.08.174
Courtin CM, Swennen K, Verjans P, Delcour JA (2009) Heat and pH stability of prebiotic arabinoxylooligosaccharides, xylooligosaccharides and fructooligosaccharides. Food Chem 112:831–837. https://doi.org/10.1016/j.foodchem.2008.06.039
Grafulin VY (2018) Xilo-oligossacarídeos - aplicação em alimentos e produção a partir de resíduoslignocelulósicos: uma revisão da literatura. Dissertation, Federal University of Rio Grande Do Sul
Czaikoski A, Lopes R, Menegalli FC (2020) Rheological behavior of cellulose nanofibers from cassava peel obtained by combination of chemical and physical processes. Carbohydr Polym 248:116744. https://doi.org/10.1016/j.carbpol.2020.116744
Andrade JC De Determinações Iodométricas. Available at: https://econtents.bc.unicamp.br/inpec/index.php/chemkeys/article/view/9623. Accessed 16 May 2022
Gangola MP, Ramadoss BR, Jaiswal S et al (2021) Faba bean meal, starch or protein fortification of durum wheat pasta differentially influence noodle composition, starch structure and in vitro digestibility. Food Chem 349:129167. https://doi.org/10.1016/j.foodchem.2021.129167
Agung A, Ratnadewi I, Budi A, Sulistyaningsih E (2016) Application of cassava peel and waste as raw materials for xylooligosaccharide production using endoxylanase from Bacillus subtilis of soil termite abdomen. Procedia Chem 18:31–38. https://doi.org/10.1016/j.proche.2016.01.007
Singh A, Bishnoi NR (2013) Comparative study of various pretreatment techniques for ethanol production from water hyacinth. Ind Crops Prod 44:283–289. https://doi.org/10.1016/j.indcrop.2012.11.026
Salihu A, Abbas O, Sallau AB, Alam MZ (2015) Agricultural residues for cellulolytic enzyme production by Aspergillus niger: effects of pretreatment. 3 Biotech 5:1101–1106
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Biomass Anal Technol Team Lab Anal Proced 1–15. https://www.nrel.gov/docs/gen/fy13/42618.pdf. Accessed 15 Aug 2022
Pereira GN, Cesca K, Vieira Cubas AL, de Oliveira D (2021) Use of non-thermal plasma in lignocellulosic materials: a smart alternative. Trends Food Sci Technol 109:365–373. https://doi.org/10.1016/j.tifs.2021.01.047
Ávila PF, Martins M, Goldbeck R (2020) Enzymatic production of xylooligosaccharides from alkali-solubilized arabinoxylan from sugarcane straw and coffee husk. Bioenergy Res 14:739–751. https://doi.org/10.1007/s12155-020-10188-7
Maniglia BC (2017) Aproveitamento e resíduos agroindustriais para elaboração de filmes biodegradáveis. Thesis, University of São Paulo
Young CG, Volaric SS (2020) Synthesis and iodometric analysis of the polyiodide salt (NMe4)[I5]. J Chem Educ 97:1117–1119. https://doi.org/10.1021/acs.jchemed.9b00852
Patrizi N, Bruno M, Saladini F et al (2020) Sustainability assessment of biorefinery systems based on two food residues in Africa. Front Sustain Food Syst 4:1–13. https://doi.org/10.3389/fsufs.2020.522614
Collares RM (2011) Otimização do processo de hidrólise da mandioca “in natura”, com o uso de enzimasamilolíticas e pectinolítica. Dissertation, Federal University of Santa Maria
Karimi R, Azizi MH, Xu Q et al (2018) Enzymatic removal of starch and protein during the extraction of dietary fiber from barley bran. J Cereal Sci 83:259–265. https://doi.org/10.1016/j.jcs.2018.07.012
Castro HF (2009) Processos químicos industriais II - papel e celulose. USP. 1:30. https://sistemas.eel.usp.br/docentes/arquivos/5840556/434/apostila4papelecelulose.pdf. Accessed 12 Aug 2022
Awoyale AA, Lokhat D, Eloka-Eboka AC (2021) Experimental characterization of selected Nigerian lignocellulosic biomasses in bioethanol production. Int J Ambient Energy 42:1343–1351. https://doi.org/10.1080/01430750.2019.1594375
Ona JI, Halling PJ, Ballesteros M (2019) Enzyme hydrolysis of cassava peels: treatment by amylolytic and cellulolytic enzymes. Biocatal Biotransformation 37:77–85. https://doi.org/10.1080/10242422.2018.1551376
Aruwajoye GS, Faloye FD, Kana EG (2017) Soaking assisted thermal pretreatment of cassava peels wastes for fermentable sugar production: process modelling and optimization. Energy Convers Manag 150:558–566. https://doi.org/10.1016/j.enconman.2017.08.046
Moshi AP, Temu SG, Nges IA et al (2015) Combined production of bioethanol and biogas from peels of wild cassava Manihot glaziovii. Chem Eng J 279:297–306. https://doi.org/10.1016/j.cej.2015.05.006
Ronko LZ, Travalini AP, Demiate IM (2020) Amido e bagaço de mandioca (Manihot esculenta C.): obtenção e caracterização de diferentes variedades. Rev Bras Tecnol Agroindustrial 14:2962–2982. https://doi.org/10.3895/rbta.v1n1.10799
Amalia AV, Fibriana F, Widiatningrum T, Hardianti RD (2021) Bioconversion and valorization of cassava-based industrial wastes to bioethanol gel and its potential application as a clean cooking fuel. Biocatal Agric Biotechnol 35:102093. https://doi.org/10.1016/j.bcab.2021.102093
Sudha A, Sivakumar V, Sangeetha V, Priyenka Devi KS (2018) Physicochemical treatment for improving bioconversion of cassava industrial residues. Environ Prog Sustain Energy 37:577–583. https://doi.org/10.1002/ep.12702
Sindhu R, Kuttiraja M, Binod P et al (2014) Physicochemical characterization of alkali pretreated sugarcane tops and optimization of enzymatic saccharification using response surface methodology. Renew Energy 62:362–368. https://doi.org/10.1016/j.renene.2013.07.041
Nikzad M, Movagharnejad K, Talebnia F et al (2015) Modeling of alkali pretreatment of rice husk using response surface methodology and artificial neural network. Chem Eng Commun 202:728–738. https://doi.org/10.1080/00986445.2013.871707
Fang L, Su Y, Wang P et al (2022) Co-production of xylooligosaccharides and glucose from birch sawdust by hot water pretreatment and enzymatic hydrolysis. Bioresour Technol 348:126795. https://doi.org/10.1016/j.biortech.2022.126795
Shahabazuddin M, Sarat Chandra T, Meena S et al (2018) Thermal assisted alkaline pretreatment of rice husk for enhanced biomass deconstruction and enzymatic saccharification: physico-chemical and structural characterization. Bioresour Technol 263:199–206. https://doi.org/10.1016/j.biortech.2018.04.027
Rai P, Pandey A, Pandey A (2019) Optimization of sugar release from banana peel powder waste (BPPW) using Box-Behnken design (BBD): BPPW to biohydrogen conversion. Int J Hydrogen Energy 44:25505–25513. https://doi.org/10.1016/j.ijhydene.2019.07.168
Pereira GN, Cesca K, Cubas ALV, Bianchet RT, Junior SEB, Zanella E, Stambuk BU, Poleto P, Oliveira D (2021) Non-thermal plasma as an innovative pretreatment technology in delignification of brewery by-product. Innov Food Sci Emerg Technol 74(102827):1–8. https://doi.org/10.1016/j.ifset.2021.102827
Wilkinson S, Smart KA, Cook DJ (2014) Optimisation of alkaline reagent based chemical pre-treatment of brewers spent grains for bioethanol production. Ind Crops Prod 62:219–227. https://doi.org/10.1016/j.indcrop.2014.08.036
Li H, Pu Y, Kumar R et al (2014) Investigation of lignin deposition on cellulose during hydrothermal pretreatment, its effect on cellulose hydrolysis, and underlying mechanisms. Biotechnol Bioeng 111:485–492. https://doi.org/10.1002/bit.25108
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2005) Determination of extractives in biomass. Biomass Anal Technol Team Lab Anal Proced 1–12. https://www.nrel.gov/docs/gen/fy08/42619.pdf. Accessed 18 Aug 2022
Chundawat SPS, Vismeh R, Sharma LN et al (2010) Multifaceted characterization of cell wall decomposition products formed during ammonia fiber expansion (AFEX) and dilute acid based pretreatments. Bioresour Technol 101:8429–8438. https://doi.org/10.1016/j.biortech.2010.06.027
Khalid MJ, Zeshan WA, Nawaz I (2019) Synergistic effect of alkaline pretreatment and magnetite nanoparticle application on biogas production from rice straw. Bioresour Technol 275:288–296. https://doi.org/10.1016/j.biortech.2018.12.051
Remli NAM, Md Shah UK, Mohamad R, Abd-Aziz S (2014) Effects of chemical and thermal pretreatments on the enzymatic saccharification of rice straw for sugars production. BioResources 9:510–522. https://doi.org/10.15376/biores.9.1.510-522
Kamalini A, Muthusamy S, Ramapriya R et al (2018) Optimization of sugar recovery efficiency using microwave assisted alkaline pretreatment of cassava stem using response surface methodology and its structural characterization. J Mol Liq 254:55–63. https://doi.org/10.1016/j.molliq.2018.01.091
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Szczerbowski D, Pitarelo AP, Zandoná Filho A, Ramos LP (2014) Sugarcane biomass for biorefineries: comparative composition of carbohydrate and non-carbohydrate components of bagasse and straw. Carbohydr Polym 114:95–101. https://doi.org/10.1016/j.carbpol.2014.07.052
Gupta M, Bangotra R, Sharma S et al (2022) Bioprocess development for production of xylooligosaccharides prebiotics from sugarcane bagasse with high bioactivity potential. Ind Crops Prod 178:114591. https://doi.org/10.1016/j.indcrop.2022.114591
Aachary AA, Prapulla SG (2009) Value addition to corncob: production and characterization of xylooligosaccharides from alkali pretreated lignin-saccharide complex using Aspergillus oryzae MTCC 5154. Bioresour Technol 100:991–995. https://doi.org/10.1016/j.biortech.2008.06.050
Banerjee S, Patti AF, Ranganathan V, Arora A (2019) Hemicellulose based biorefinery from pineapple peel waste: xylan extraction and its conversion into xylooligosaccharides. Food Bioprod Process 117:38–50. https://doi.org/10.1016/j.fbp.2019.06.012
Khat-udomkiri N, Sivamaruthi BS, Sirilun S, Lailerd N, Peerajan S, Chaiyasut C (2018) Optimization of alkaline pretreatment and enzymatic hydrolysis for the extraction of xylooligosaccharide from rice husk. AMB Express 8(115):1–10. https://doi.org/10.1186/s13568-018-0645-9
Jnawali P, Kumar V, Tanwar B et al (2018) Enzymatic production of xylooligosaccharides from brown coconut husk treated with sodium hydroxide. Waste Biomass Valor 9:1757–1766. https://doi.org/10.1007/s12649-017-9963-4
Cebin AV, Ralet MC, Vigouroux J, Karača S, Martinić A, Komes D, Bonnin E (2021) Valorisation of walnut shell and pea pod as novel sources for the production of xylooligosaccharides. Carbohydr Polym 263(117932):1–11. https://doi.org/10.1016/j.carbpol.2021.117932
Ho AL, Kosik O, Lovegrove A et al (2018) In vitro fermentability of xylo-oligosaccharide and xylo-polysaccharide fractions with different molecular weights by human faecal bacteria. Carbohydr Polym 179:50–58. https://doi.org/10.1016/j.carbpol.2017.08.077
Monteiro CRM, Ávila PF, Pereira MAF, Pereira GM, Bordignon SE, Zanella E, Stambuk BU, Oliveira D, Goldbeck R, Poleto P (2021) Hydrothermal treatment on depolymerization of hemicellulose of mango seed shell for the production of xylooligosaccharides. Carbohydr Polym 253(117274):1–8. https://doi.org/10.1016/j.carbpol.2020.117274
Cao L, Yu IKM, Liu Y et al (2018) Lignin valorization for the production of renewable chemicals: state-of-the-art review and future prospects. Bioresour Technol 269:465–475. https://doi.org/10.1016/j.biortech.2018.08.065
Plaza M, Turner C (2015) Pressurized hot water extraction of bioactives. TrAC - Trends Anal Chem 71:39–54. https://doi.org/10.1016/j.trac.2015.02.022
Forsan CF, Paz Cedeño FR, Masarin F, Brienzo M (2021) Xylooligosaccharides production by optimized autohydrolysis, sulfuric and acetic acid hydrolysis for minimum sugar degradation production. Bioact Carbohydrates Diet Fibre 26(100268):1–12. https://doi.org/10.1016/j.bcdf.2021.100268
Yuan Y, Jiang B, Chen H et al (2021) Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol Biofuels 14:1–20. https://doi.org/10.1186/s13068-021-02054-1
Acknowledgements
The authors acknowledge the financial support of Foundation for the Research and Innovation Support Foundation of Santa Catarina State (FAPESC) for the founding Programa de Apoio a Núcleos Emergentes (PRONEM) 2020TR731 and by the scholarship of co-author Eduardo Zanella. Many thanks also for Coordination for the Improvement of Higher Education Personnel (CAPES)—Institutional Program for Internationalization (PRINT), project numbers 88887.310560/2018-00 and 88887.310727/2018-00.
Author information
Authors and Affiliations
Contributions
William Rogoski: conceptualization, methodology, software, writing—original draft, writing—article and editing, and formal analysis; Gabriela Nayana Pereira: formal analysis and conceptualization; Karina Cesca: conceptualization, project administration; Moisés Amancio da Silva: formal analysis; Eduardo Zanella: formal analysis; Boris U. Stambuk: formal analysis; Patrícia F. Ávila: formal analysis; and Rosana Goldbeck: formal analysis. Débora de Oliveira: term, supervision, funding acquisition; Cristiano José de Andrade: term, supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rogoski, W., Pereira, G.N., Cesca, K. et al. Production of cassava peel-based xylooligosaccharides using endo-1,4-β-xylanase from Trichoderma longibrachiatum: the effect of alkaline pretreatment. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03287-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03287-2