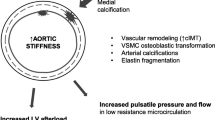
Abstract
Background
Pulse wave velocity (PWV) is a measure of arterial stiffness. We investigated PWV and blood pressure (BP) to determine to what extent BP changes contribute to arterial stiffness, and secondly, to identify influencing factors on BP in children after kidney transplantation.
Methods
Seventy children ≥ 2.5 years post-transplantation with at least two PWV measurements were included. Changes of systolic (Δ SBP) and diastolic BP (Δ DBP) were classified into “stable/decreasing,” “1–10 mmHg increase,” and “ > 10 mmHg increase.” Linear mixed modeling for PWV z-score (PWVz) adjusted either for Δ SBP or Δ DBP was performed. An extended dataset with monthly entries of BP, immunosuppression, and creatinine was obtained in 35 participants over a median of 74 months to perform linear mixed modeling for SBP and DBP.
Results
PWVz increased with a rate of 0.11/year (95% CI 0.054 to 0.16). Compared to participants with stable BP, those with 1–10-mmHg SBP and DBP increase showed a higher PWVz of 0.59 (95% CI 0.046 to 1.13) and 0.86 (95% CI 0.43 to 1.30), respectively. A > 10-mmHg BP increase was associated with an even higher PWVz (SBP β = 0.78, 95% CI 0.22 to 1.34; DBP β = 1.37, 95% CI 0.80 to 1.94). Female sex and participants with lower eGFR showed higher PWVz.
In the extended analysis, DBP was positively associated with cyclosporin A and everolimus trough levels.
Conclusions
A higher increase of PWV is seen in patients with greater BP increase, with higher cyclosporin A and everolimus trough levels associated with higher BP. This emphasizes the role of BP as a modifiable risk factor for the improvement of cardiovascular outcome after transplantation.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Introduction
Cardiovascular mortality in children with kidney failure is very high. About 30% of deaths in children on dialysis and 25% in children undergoing kidney transplantation (KTx) are due to cardiovascular events [1]. Data from the Australian and New Zealand Dialysis and Transplant Registry show an even higher cardiovascular mortality rate after KTx with 40% [2]. Importantly, mortality due to cardiovascular causes is higher than mortality due to non-functioning grafts [3].
The scarcity of cardiovascular mortality as well as major cardiovascular events in pediatric patients with chronic kidney disease (CKD) makes these endpoints unsuitable for studies in children. Arteriosclerosis, a marker for subclinical cardiovascular damage, is the process where elastic fibers are replaced by stiffer collagenous fibers after they break down due to daily wear and tear, which is eventually followed by the calcification of the media layer. This process is a major element in the stiffening of large arteries, which can be assessed by pulse wave velocity (PWV). PWV is highly predictive of major cardiovascular events and cardiovascular mortality in adults [4]. Although its predictive value has not been demonstrated in children, PWV measurement in the setting of clinical studies is recommended by the American Heart Association [5]. The procedure can be performed non-invasively and is highly reproducible in children [6, 7]. Furthermore, PWV is shown to be elevated in pediatric patients with CKD as well as after KTx, when compared to healthy peers [8,9,10,11,12].
A strong dependency between arterial stiffness and blood pressure (BP) is established for healthy children [13, 14] and children with CKD of all stages [10, 15,16,17], as well as in children after KTx [11, 18, 19]. In adults, not only the given BP levels but also the dynamic changes of BP over time have been shown to affect cardiovascular events [20]. In adult KTx recipients, a temporary BP increase is associated not only with worse patient survival, but also with inferior graft survival [21]. In fact, a 10-mmHg BP increase in patients with a systolic BP of > 140 mmHg is associated with a 12% higher risk of graft failure [22], and a 20-mmHg increase from a baseline systolic BP is associated with a 32% higher risk of subsequent cardiovascular disease [23]. In healthy male adults, the annual change in BP is positively associated with the progression of arterial stiffness [24], while a decrease in BP is associated with a lower PWV in patients with hemodialysis [25]. The association between BP dynamics and PWV has not been described in children yet.
We aimed to decipher the association between BP changes and progression of arterial stiffness. Our specific goal was to determine a clinically applicable BP range that may aggravate arterial stiffness in pediatric KTx recipients late after transplantation. By using an extended dataset with monthly entries over a period of up to 9 years, we aimed to further explore which factors are potentially associated with higher BP in a sub-cohort of our patients.
Methods
Subjects and study design
From January 2011 to August 2019, 124 pediatric KTx recipients from three German pediatric nephrology units (University Hospital Essen n = 41, University Medical Center Hamburg-Eppendorf n = 22, and Hannover Medical School n = 61) were enrolled in this prospective multicenter cohort study [18] after obtaining written informed consent from parents or caregivers. While per study protocol the only inclusion criteria were functioning kidney graft and age between 6 and 18 years, this analysis focused on patients with two or more follow-up visits ≥ 2.5 years after transplantation. The exclusion criteria were active systemic vasculitis, renal vascular anomalies, coexisting primary cardiovascular anomalies, and anomalies of the limbs preventing diagnostic procedures (which did not apply to any participant). The study was approved by each center’s ethics committee and fully adheres to the Declaration of Helsinki.
Primary dataset
Aortic PWV was assessed every 2 years by two jointly trained investigators as previously reported [7] using the oscillometric Vicorder device (SMT Medical, Würzburg, Germany) in accordance with the recommendation of the Task Force III on clinical applications of arterial stiffness [26]. In short, three consecutive measurements with a length of at least 10 heart beats were performed in a supine position after 10 min of rest and then averaged. Blood and urine samples were obtained on the PWV measurement day and analyzed by a central laboratory (Synlab, Heidelberg, Germany), including whole blood count, creatinine, cystatin C, urea, and immunosuppressive trough levels. Creatinine was analyzed enzymatically, and immunosuppressive trough levels were analyzed using liquid chromatography and tandem mass spectrometry. Information on underlying disease, transplantation and dialysis history, and medication were obtained from the medical charts. Antihypertensive medication was classified into angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists, β-blockers, calcium channel blockers, diuretics, vasodilators, α-blockers, and central acting agents. Measurement of office BP at each unit was performed on the same day by a pediatric nurse on the right arm in a seated position after 5 min of rest, using a validated oscillometric device (Hannover and Essen: Dinamap V100, GE Medical Systems, Chicago, IL, USA; Hamburg: Mindray VS-900, Mindray Bio-Medical Electronics, Shenzhen, China) with an appropriately sized cuff.
Extended dataset
An extended dataset from the Hannover patients comprising monthly BP measurements, height, BMI, creatinine, and immunosuppressive trough levels was collected from medical records.
Endpoints
As our primary endpoint, PWV measurements were transformed to standardized score (z-score) adjusted for sex and height [6]. Further endpoints in our extended dataset were systolic and diastolic BP.
Covariates
We calculated age-, height-, and sex-standardized z-score for BP [27, 28], as well as age- and sex-standardized z-score for BMI. The estimated glomerular filtration rate (eGFR) was calculated using the Schwartz formula [29]. Immunosuppressive agents were classified as calcineurin inhibitors (CNI, including cyclosporin A or tacrolimus), mycophenolate mofetil (MMF), mammalian target of rapamycin inhibitors (m-TOR, including everolimus, and sirolimus), and steroids. Underlying primary kidney disease was classified as either congenital anomalies of the kidney and urinary tract (CAKUT) or primary kidney disease other than CAKUT (non-CAKUT).
The change of BP was calculated as the difference between the BP at each given visit and the BP at the first visit at ≥ 2.5 years after transplantation (baseline visit). We classified the change of BP into three categories: “stable/decrease,” “1–10 mmHg increase,” and “ > 10 mmHg increase.” Hypertensive BP was defined as systolic and/or diastolic BP ≥ 95th percentile (z-score ≥ 1.645) for patients with age < 16 years, or as a systolic/diastolic BP ≥ 140/90 mmHg for those ≥ 16 years old, or if the patient was on antihypertensive medication [28]. A non-hypertensive BP without antihypertensive medication was classified as “non-hypertension.” Further classification included “controlled hypertension” (non-hypertensive BP and treated), “uncontrolled hypertension” (hypertensive BP and treated), and “untreated hypertension” (hypertensive BP and untreated).
Statistical analysis
Continuous variables are summarized by means (± standard deviation, SD) and categorical variables by frequencies and percentages. Differences between two or more independent groups were assessed using ANOVA or χ2 test for categorical variables. To investigate the effect of BP changes on PWVz, we performed a multivariable linear mixed effect modeling (mixed modeling) for PWV z-score (PWVz) at follow-up visits (after baseline visits), adjusted for sex [6, 12, 30], underlying disease, and baseline value of BP, as well as age [6], BMI [31], and eGFR [32] at the respective follow-up visit. Using the extended dataset, two mixed model analyses were performed, each for systolic and diastolic BP to investigate the associations with immunosuppressive trough levels (cyclosporin A, tacrolimus, everolimus), adjusted for sex [30, 33], and underlying disease, as well as age [30], BMI [34], and eGFR at the respective visits. Random intercept and slope to account for inter-individual variation and time since baseline visit as repeated effect to account for intra-individual variation were included. A two-tailed p-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Primary dataset
Of 124 pediatric KTx recipients, 70 (n = 43 males) with at least two visits were included, resulting in 211 observations. At baseline, the age was 12.6 ± 3.2 years and elapsed time since transplantation was 6.1 ± 3.1 years. Participants were followed up for 4.0 ± 2.0 years with a maximum follow-up of 8 years. Fifty-one participants were transplanted after prior dialysis. The mean eGFR at the baseline visit was 56.6 ± 26.5 mL/min/1.73 m2. Detailed participant characteristics are given in Table 1. Supplemental Figure S1 provides the flow diagram of the study population in the primary dataset.
Baseline systolic and diastolic BP z-scores were 0.86 ± 0.92 and 0.64 ± 0.85, respectively. Arterial hypertension was present in 88% of patients (69% controlled, 16% uncontrolled, and 3% untreated hypertension). The proportion of hypertension remained high over time, and the proportion of uncontrolled hypertension increased to 28% at the first follow-up visit (Fig. 1a) and could also be observed for further follow-up visits (data not shown). Figure 1b and c show the proportion of isolated systolic and isolated diastolic hypertension, respectively. Eighty-six percent of the participants received antihypertensive medication, which remained stable over time. The majority received a combination of three or more antihypertensive drugs (Supplemental Table S1), with ACE inhibitors, calcium channel blockers, and \(\beta\)-blockers as the most frequently used agents.
Of all participants, 97% had a combination of two (76%) or three (21%) immunosuppressive agents. The majority received a CNI-based regimen (97%), with CNI-mTOR, CNI-MMF, and CNI-steroids as the most commonly used combinations (Supplemental Table S2). At the baseline visit, the mean trough levels for cyclosporin A, tacrolimus, and everolimus were at 92.3 ± 29.3, 5.1 ± 1.8, and 3.9 ± 0.95 µg/L, respectively (Supplemental Table S2).
The course of PWVz
The mean of PWVz was 0.10 ± 1.31 at baseline and increased to 0.52 ± 1.26 after 2 years (Supplemental Table S3). The increase of PWVz was also demonstrated after adjustment for time since KTx with a rate of 0.11 per year (95% CI 0.054 to 0.16) (Fig. 2).
PWV and the changes of BP
Of 70 participants, 5 were excluded from the models due to missing BP at baseline and at following visits, and eGFR at following visits (Supplemental Fig. S1). In the model for PWVz and changes of systolic BP, when compared to participants with a stable/decreasing systolic BP, those with a systolic BP increase between 1 and 10 mmHg showed a higher PWVz increase of 0.59 (95% CI 0.046 to 1.13). A systolic BP increase of > 10 mmHg was associated with an even higher PWVz increase of 0.78 (95% CI 0.22 to 1.34). Significant associations between female sex, non-CAKUT underlying disease, and a lower eGFR with a higher PWVz were shown (Table 2).
The model for PWVz and changes in diastolic BP (Table 3) showed associations with even higher PWVz increases. Compared to children with stable/decreasing diastolic BP, children displaying an increase in diastolic BP between 1 and 10 mmHg showed a higher PWVz of 0.86 (95% CI 0.43 to 1.30). An increase in diastolic BP of > 10 mmHg was associated with a higher PWVz of 1.37 (95% CI 0.80 to 1.94). A higher PWVz was also associated with a higher baseline diastolic BP and female sex.
In both models adjusted for changes in systolic and diastolic BP, age and BMI did not reveal a significant association with PWVz (Tables 2 and 3). We did not find associations between PWVz and immunosuppressive trough levels (Supplemental Table S4). Figure 3 illustrates the different PWVz slopes according to the defined categories of BP increases (stable/decreasing, 1–10 mmHg or > 10 mmHg) in systolic or diastolic BP.
Illustration for the slopes of PWV z-score according to the defined categories of BP increases (stable/decreasing, 1–10 mmHg, or > 10 mmHg) in systolic or diastolic BP. The slopes are calculated according to the mixed models presented in Tables 2 and 3. Gray lines show the slopes for stable/decreasing systolic and diastolic BP. Black dotted lines show the slopes for systolic and diastolic BP increase of 1–10 mmHg. Black solid lines show the slopes for systolic and diastolic BP increase of > 10 mmHg. Abbreviations: DBP, diastolic blood pressure; PWV, pulse wave velocity; SBP, systolic blood pressure
Extended data analysis: blood pressure and immunosuppressive trough levels
We used an extended dataset to evaluate the importance of immunosuppression on BP. We retrieved data on systolic and diastolic BP, eGFR, and immunosuppressive trough levels from 35 participants (n = 20 males) comprising 2137 observations. The participants’ characteristics of the extended dataset are given in Supplemental Table S5. Tables 4 and 5 show the models for systolic and diastolic BP.
We did not find an association between immunosuppressive trough levels and systolic BP. Systolic BP increased with age. A higher BMI was associated with a higher systolic BP (Table 4).
In the model for diastolic BP, we found associations with higher trough levels of cyclosporin A (β = 0.043, 95% CI 0.021 to 0.065) and everolimus (β = 0.42, 95% CI 0.12 to 0.72). Diastolic BP increased with age (Table 5).
Discussion
This prospective multicenter study on pediatric KTx recipients investigated PWV and its changes in dependence on BP changes at more than 2.5 years after transplantation. We demonstrated an increase in PWV late after transplantation that is associated with increasing BP values. We did not find associations between immunosuppressive trough levels and PWV. However, higher trough levels of cyclosporin A and everolimus were associated with higher diastolic BP values in our extended data analysis.
KTx resolves uremia, fluid overload, and other metabolic abnormalities, as reflected by the improvement of arterial stiffness observed in KTx recipients compared to patients on dialysis [11, 35, 36]. However, cardiovascular morbidity in this population remains high due to unresolved or newly developed risk factors such as hypertension, dyslipidemia, and obesity, exemplified by the high prevalence of post-transplant metabolic syndrome [37]. Compared to healthy children, arterial stiffness in pediatric KTx recipients with a functioning graft is higher [19] and further increases after transplantation [12, 38] indicating early development of arteriosclerosis. BP is a strong determinant for arterial stiffness in healthy children [13, 14] as well as those with CKD [10, 11, 18, 19]. In our longitudinal setup, we were able to identify the association between particular ranges of BP increases and the progression of arterial stiffness. An increase of 10 mmHg or more in systolic or diastolic BP was associated with a higher increase in arterial stiffness. Arterial hypertension is still highly prevalent and poorly controlled after KTx [39,40,41,42]. Whether strict BP control might reverse the pathological increase of arterial stiffness after KTx merits further research.
Our data did not show an association between different immunosuppressive drug trough levels and arterial stiffness. Randomized controlled trials in adults investigating the effect of different immunosuppressive regimens on arterial stiffness showed inconsistent results. A randomized controlled trial in 27 KTx recipients who were either switched from a CNI-based therapy to everolimus or remained on CNI therapy at 6 months after transplantation showed stable PWV in the everolimus group and a PWV increase in the CNI group at 15 months post-transplantation [43]. However, a larger prospective multicenter randomized controlled trial including 709 KTx recipients showed no differences in PWV between patients receiving standard therapy and those who switched to everolimus with a CNI-free regimen at 10–14 weeks after KTx [44]. We had previously demonstrated an association between the use of everolimus and higher PWV [18] and an association between higher tacrolimus or cyclosporin A trough levels with higher BP [42]. From our current findings, therefore, we speculate that the influence of everolimus and cyclosporin A on PWV is mediated through their effect on BP.
Our adjusted model suggested a possible association between female sex and a higher PWV z-score. This is consistent with our previous study demonstrating a greater vulnerability of female children with kidney failure and subsequent transplantation to develop arterial stiffness compared to males [12]. A study in adults demonstrated greater arterial stiffness in older females; however, this effect was attenuated with further adjustment for cardiovascular risk factors [45]. The possible association of lower eGFR with higher PWV is also in line with a previous study showing an association between faster eGFR decline and higher arterial stiffness [32]. The higher PWV in participants with underlying kidney diseases other than CAKUT might be explained by the association with a faster decline of kidney function shown in glomerular kidney disease [46] prior to transplantation. These exploratory observed associations between the covariates other than the primary exposure with the outcome variables are intriguing and warrant further investigations.
This study has some limitations. Longitudinal studies are subject to attrition bias; older participants in our cohort tended to have fewer follow-up visits due to their transition to adult care. However, PWVz did not differ between participants with 2 visits and those with > 2 visits. In addition, age was included in the mixed models. Our focus was to show how BP changes affect the PWV irrespective of the treatment strategies; we deliberately did not include variables indicating the use of antihypertensive medication. The lack of pre-KTx data in our dataset limits the exploration of potentially important factors, e.g., kidney functional decline during CKD progression. The data for the extended analysis was only available for Hannover patients. PWV and BP are highly predictive of cardiovascular outcome, but they are surrogate markers and not hard cardiovascular endpoints, such as myocardial infarction. We used single automated office BP measurements in our analysis. The nature of the observational study design does not allow to infer causality. Our study shows several strengths. The multicenter approach allowed us to include a high number of pediatric KTx recipients from three large German centers. All participants were followed longitudinally with standardized cardiovascular assessments. Our pediatric cohort is not confounded by age-related comorbidities as is usually the case in studies in adult populations, providing a clearer insight on cardiovascular changes.
Conclusion
A higher burden of arterial stiffening with increasing BP of more than 10 mmHg as well as the association between higher cyclosporin A and everolimus trough levels with BP indicates the importance of modifiable risk factors for cardiovascular outcome after transplantation. It also emphasizes that there is room for improvement in BP control and highlights that the choice of immunosuppressive therapy can interfere with BP.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mitsnefes MM (2012) Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23:578–585
Francis A, Johnson DW, Melk A, Foster BJ, Blazek K, Craig JC, Wong G (2020) Survival after kidney transplantation during childhood and adolescence. Clin J Am Soc Nephrol 15:392–400
Harambat J, van Stralen KJ, Kim JJ, Tizard EJ (2012) Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27:363–373
Ferreira JP, Girerd N, Pannier B, Rossignol P, London GM (2017) High pulse-wave velocity defines a very high cardiovascular risk cohort of dialysis patients under age 60. Am J Nephrol 45:72–81
Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, Jacobson M, Mahoney L, Mietus-Snyder M, Rocchini A, Steinberger J, McCrindle B (2009) Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension 54:919–950
Thurn D, Doyon A, Sozeri B, Bayazit AK, Canpolat N, Duzova A, Querfeld U, Schmidt BM, Schaefer F, Wuhl E, Melk A (2015) Aortic pulse wave velocity in healthy children and adolescents: reference values for the Vicorder device and modifying factors. Am J Hypertens 28:1480–1488
Kracht D, Shroff R, Baig S, Doyon A, Jacobi C, Zeller R, Querfeld U, Schaefer F, Wuhl E, Schmidt BM, Melk A (2011) Validating a new oscillometric device for aortic pulse wave velocity measurements in children and adolescents. Am J Hypertens 24:1294–1299
Kis E, Cseprekal O, Horvath Z, Katona G, Fekete BC, Hrapka E, Szabo A, Szabo AJ, Fekete A, Reusz GS (2008) Pulse wave velocity in end-stage renal disease: influence of age and body dimensions. Pediatr Res 63:95–98
Covic A, Mardare N, Gusbeth-Tatomir P, Prisada O, Sascau R, Goldsmith DJ (2006) Arterial wave reflections and mortality in haemodialysis patients–only relevant in elderly, cardiovascularly compromised? Nephrol Dial Transplant 21:2859–2866
Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, Niemirska A, Sozeri B, Thurn D, Anarat A, Ranchin B, Litwin M, Caliskan S, Candan C, Baskin E, Yilmaz E, Mir S, Kirchner M, Sander A, Haffner D, Melk A, Wuhl E, Shroff R, Querfeld U (2017) Cardiovascular phenotypes in children with CKD: the 4C study. Clin J Am Soc Nephrol 12:19–28
Schmidt BMW, Sugianto RI, Thurn D, Azukaitis K, Bayazit AK, Canpolat N, Eroglu AG, Caliskan S, Doyon A, Duzova A, Karagoz T, Anarat A, Deveci M, Mir S, Ranchin B, Shroff R, Baskin E, Litwin M, Ozcakar ZB, Buscher R, Soylemezoglu O, Dusek J, Kemper MJ, Matteucci MC, Habbig S, Laube G, Wuhl E, Querfeld U, Sander A, Schaefer F, Melk A (2018) Early effects of renal replacement therapy on cardiovascular comorbidity in children with end-stage kidney disease: findings from the 4C-T study. Transplantation 102:484–492
Sugianto RI, Memaran N, Schmidt BMW, Doyon A, Thurn-Valsassina D, Alpay H, Anarat A, Arbeiter K, Azukaitis K, Bayazit AK, Bulut IK, Caliskan S, Canpolat N, Duzova A, Gellerman J, Harambat J, Homeyer D, Litwin M, Mencarelli F, Obrycki L, Paripovic D, Ranchin B, Shroff R, Tegtbur U, von der Born J, Yilmaz E, Querfeld U, Wühl E, Schaefer F, Melk A (2022) Findings from 4C-T study demonstrate an increased cardiovascular burden in girls with end stage kidney disease and kidney transplantation. Kidney Int 101:585–596
Stabouli S, Kollios K, Nika T, Chrysaidou K, Tramma D, Kotsis V (2020) Ambulatory hemodynamic patterns, obesity, and pulse wave velocity in children and adolescents. Pediatr Nephrol 35:2335–2344
Li S, Chen W, Srinivasan SR, Berenson GS (2004) Childhood blood pressure as a predictor of arterial stiffness in young adults: the Bogalusa Heart Study. Hypertension 43:541–546
Savant JD, Betoko A, Meyers KE, Mitsnefes M, Flynn JT, Townsend RR, Greenbaum LA, Dart A, Warady B, Furth SL (2017) Vascular stiffness in children with chronic kidney disease. Hypertension 69:863–869
Tasdemir M, Eroglu AG, Canpolat N, Konukoglu D, Agbas A, Sevim MD, Caliskan S, Sever L (2016) Cardiovascular alterations do exist in children with stage-2 chronic kidney disease. Clin Exp Nephrol 20:926–933
Duzova A, KarabayBayazit A, Canpolat N, Niemirska A, Kaplan Bulut I, Azukaitis K, Karagoz T, Oguz B, Erdem S, Anarat A, Ranchin B, Shroff R, Djukic M, Harambat J, Yilmaz A, Yildiz N, Ozcakar B, Buscher A, Lugani F, Wygoda S, Tschumi S, Zaloszyc A, Jankauskiene A, Laube G, Galiano M, Kirchner M, Querfeld U, Melk A, Schaefer F, Wuhl E, 4C Study Consortium (2019) Isolated nocturnal and isolated daytime hypertension associate with altered cardiovascular morphology and function in children with chronic kidney disease: findings from the Cardiovascular Comorbidity in Children with Chronic Kidney Disease study. J Hypertens 37:2247–2255
Borchert-Morlins B, Thurn D, Schmidt BMW, Buscher AK, Oh J, Kier T, Bauer E, Baig S, Kanzelmeyer N, Kemper MJ, Buscher R, Melk A (2017) Factors associated with cardiovascular target organ damage in children after renal transplantation. Pediatr Nephrol 32:2143–2154
Briese S, Claus M, Querfeld U (2008) Arterial stiffness in children after renal transplantation. Pediatr Nephrol 23:2241–2245
Sesso HD, Stampfer MJ, Rosner B, Gaziano JM, Hennekens CH (2000) Two-year changes in blood pressure and subsequent risk of cardiovascular disease in men. Circulation 102:307–312
Opelz G, Dohler B (2005) Improved long-term outcomes after renal transplantation associated with blood pressure control. Am J Transplant 5:2725–2731
Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, O’Shaughnessy EA, Dahl DC, Silkensen JR, Sahadevan M, Snyder JJ (2004) Hypertension after kidney transplantation. Am J Kidney Dis 43:1071–1081
Carpenter MA, John A, Weir MR, Smith SR, Hunsicker L, Kasiske BL, Kusek JW, Bostom A, Ivanova A, Levey AS, Solomon S, Pesavento T, Weiner DE (2014) BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation trial. J Am Soc Nephrol 25:1554–1562
Guo J, Fujiyoshi A, Masaki K, Vishnu A, Kadota A, Barinas-Mitchell EJ, Hisamatsu T, Ahuja V, Takashima N, Evans RW, Willcox BJ, Miura K, Rodriguez B, Ueshima H, Kuller LH, Sekikawa A (2017) The role of initial and longitudinal change in blood pressure on progression of arterial stiffness among multiethnic middle-aged men. J Hypertens 35:111–117
Tian JP, Du FH, Cheng LT, Wang T (2009) Residual renal function and arterial stiffness mediated the blood pressure change during interdialytic weight gain in hemodialysis patients. Hemodial Int 13:479–486
Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C (2002) Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens 15:445–452
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W, Redon J, Schaefer F, Seeman T, Sinha M, Stabouli S, Webb NJ, Wuhl E, Zanchetti A (2016) 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34:1887–1920
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Shen W, Zhang T, Li S, Zhang H, Xi B, Shen H, Fernandez C, Bazzano L, He J, Chen W (2017) Race and sex differences of long-term blood pressure profiles from childhood and adult hypertension: the Bogalusa Heart Study. Hypertension 70:66–74
Liu Y, Yan Y, Yang X, Li S, Bazzano L, He J, Chen W (2019) Long-term burden of higher body mass index and adult arterial stiffness are linked predominantly through elevated blood pressure. Hypertension 73:229–234
Chen SC, Chang JM, Liu WC, Tsai YC, Tsai JC, Hsu PC, Lin TH, Lin MY, Su HM, Hwang SJ, Chen HC (2011) Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol 6:724–732
Campistol JM, Romero R, Paul J, Gutierrez-Dalmau A (2004) Epidemiology of arterial hypertension in renal transplant patients: changes over the last decade. Nephrol Dial Transplant 19(Suppl 3):iii62–iii66
Wang M, Kelishadi R, Khadilkar A, Mi Hong Y, Nawarycz T, Krzywinska-Wiewiorowska M, Aounallah-Skhiri H, Esmaeil Motlagh M, Soon Kim H, Khadilkar V, Krzyzaniak A, Ben Romdhane H, Heshmat R, Chiplonkar S, Stawinska-Witoszynska B, El Ati J, Qorbani M, Kajale N, Traissac P, Ostrowska-Nawarycz L, Ardalan G, Ekbote V, Yang L, Zhao M, Liu X, Liang Y, Xi B (2020) Body mass index percentiles and elevated blood pressure among children and adolescents. J Hum Hypertens 34:319–325
Tawadrous H, Kamran H, Salciccioli L, Schoeneman MJ, Lazar J (2012) Evaluation of arterial structure and function in pediatric patients with end-stage renal disease on dialysis and after renal transplantation. Pediatr Transplant 16:480–485
Cseprekal O, Kis E, Schaffer P, Othmane Tel H, Fekete BC, Vannay A, Szabo AJ, Remport A, Szabo A, Tulassay T, Reusz GS (2009) Pulse wave velocity in children following renal transplantation. Nephrol Dial Transplant 24:309–315
Blote R, Memaran N, Borchert-Morlins B, Thurn-Valsassina D, Goldschmidt I, Beier R, Sauer M, Muller C, Sarganas G, Oh J, Buscher R, Kemper MJ, Sugianto RI, Epping J, Schmidt BMW, Melk A (2019) Greater susceptibility for metabolic syndrome in pediatric solid organ and stem cell transplant recipients. Transplantation 103:2423–2433
Lilien MR, Stroes ES, Op’t Roodt J, de Jongh S, Schroder CH, Koomans HA (2003) Vascular function in children after renal transplantation. Am J Kidney Dis 41:684–691
Stabouli S, Printza N, Dotis J, Gkogka C, Kollios K, Kotsis V, Papachristou F (2016) Long-term changes in blood pressure after pediatric kidney transplantation. Am J Hypertens 29:860–865
Seeman T, Simkova E, Kreisinger J, Vondrak K, Dusek J, Gilik J, Feber J, Dvorak P, Janda J (2006) Control of hypertension in children after renal transplantation. Pediatr Transplant 10:316–322
Sinha MD, Kerecuk L, Gilg J, Reid CJ (2012) Systemic arterial hypertension in children following renal transplantation: prevalence and risk factors. Nephrol Dial Transplant 27:3359–3368
Sugianto RI, Schmidt BMW, Memaran N, Duzova A, Topaloglu R, Seeman T, Konig S, Dello Strologo L, Murer L, Ozcakar ZB, Bald M, Shenoy M, Buescher A, Hoyer PF, Pohl M, Billing H, Oh J, Staude H, Pohl M, Genc G, Klaus G, Alparslan C, Grenda R, Rubik J, Krupka K, Tonshoff B, Wuhl E, Melk A (2020) Sex and age as determinants for high blood pressure in pediatric renal transplant recipients: a longitudinal analysis of the CERTAIN Registry. Pediatr Nephrol 35:415–426
Seckinger J, Sommerer C, Hinkel UP, Hoffmann O, Zeier M, Schwenger V (2008) Switch of immunosuppression from cyclosporine A to everolimus: impact on pulse wave velocity in stable de-novo renal allograft recipients. J Hypertens 26:2213–2219
Holdaas H, de Fijter JW, Cruzado JM, Massari P, Nashan B, Kanellis J, Witzke O, Gutierrez-Dalmau A, Turkmen A, Wang Z, Lopez P, Bernhardt P, Kochuparampil J, van der Giet M, Murbraech K, ELEVATE Study Group (2017) Cardiovascular parameters to 2 years after kidney transplantation following early switch to everolimus without calcineurin inhibitor therapy: an analysis of the randomized ELEVATE study. Transplantation 101:2612–2620
Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR (2012) Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension 60:362–368
Pierce CB, Cox C, Saland JM, Furth SL, Munoz A (2011) Methods for characterizing differences in longitudinal glomerular filtration rate changes between children with glomerular chronic kidney disease and those with nonglomerular chronic kidney disease. Am J Epidemiol 174:604–612
Acknowledgements
We would like to thank the participants and their families for their support and participation in this study, especially for their time and adherence. Special thanks goes to the nurse and study nurse team of the pediatric nephrology unit in the Essen University Hospital, the University Medical Center-Hamburg-Eppendorf, and the Hannover Medical School. This study was made possible by grants from the German Federal Ministry of Education and Research through the Integrated Research and Treatment Center for Transplantation (reference number: 01EO0802) and the Roche Organ Transplantation Research Foundation.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Graphical abstract
(PPTX 179 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sugianto, R.I., Ostendorf, K., Bauer, E. et al. Arterial stiffness and blood pressure increase in pediatric kidney transplant recipients. Pediatr Nephrol 38, 1319–1327 (2023). https://doi.org/10.1007/s00467-022-05611-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05611-4