Abstract
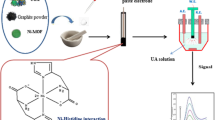
A label-free electrochemical biosensor has been developed using zinc oxide nanoflowers (ZnONFs) for the detection of uric acid concentration in human blood serum. ZnONFs have been synthesized by a hydrothermal process and characterized with several techniques such as ultraviolet–visible (UV–Vis) spectroscopy, Fourier transform infrared spectroscopy (FTIR) studies, X-ray diffraction (XRD) study, Raman spectroscopy, scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HR-TEM) and electrochemical analyzer to confirm the formation of nanoflowers and fabrication of electrode and bio-electrodes for uric acid detection. Zinc Oxide nanoflowers have been deposited onto indium–tin oxide (ITO) substrate through electrophoretic deposition technique, and the biosensor has been fabricated by immobilizing urate oxidase (UOx) enzyme onto ZnONFs/ITO electrode surfaces. Further, electrochemical studies have been performed with immobilized UOx/ZnONFs/ITO bio-electrode as a function of uric acid concentrations. It has been found that the fabricated uric acid biosensor shows a high sensitivity (10.38 μA/mM/cm2) and a limit of detection of 0.13 mM in the range of 0.005 to 1.0 mM. This study demonstrates the potential use of ZnONFs for the construction of sensitive biosensors for uric acid detection.







Similar content being viewed by others
References
M. Bhambi, G. Sumana, B.D. Malhotra and C.S. Pundir, An amperomertic uric acid biosensor based onimmobilization of uricase onto polyaniline-multiwalled carbon nanotube composite film. Artif Cells Blood Substit Immobil Biotechnol (2010). https://doi.org/10.3109/1073119100371634.
A. Umar, M.M. Rahman, M. Vaseem and Y.B. Hahn, Ultra-sensitive cholesterol biosensor based on low-temperature grown ZnO nanoparticles. Electrochemistry Communications (2009). https://doi.org/10.1016/j.elecom.2008.10.046.
F. Arellano, J. A. Sacristán, Allopurinol hypersensitivity syndrome: a review. Annals of Pharmacotherapy (1993). https://doi.org/10.1177/10600280930270031
J.A.T.L. MacLachlan, A.T.L. Wotherspoon, R.O. Ansell and C.J.W. Brooks, Cholesterol oxidase: sources, physical properties and analytical applications. The Journal of steroid biochemistry and molecular biology (2000). https://doi.org/10.1016/S0960-0760(00)00044-3.
A.K. Bhargava, H. Lal and C.S. Pundir, Discrete analysis of serum uric acid with immobilized uricase and peroxidase. Journal of biochemical and biophysical methods (1999). https://doi.org/10.1016/S0165-022X(99)00007-X.
N. Chauhan and C.S. Pundir, An amperometric uric acid biosensor based on multiwalled carbon nanotube–gold nanoparticle composite. Analytical biochemistry (2011). https://doi.org/10.1016/j.ab.2011.02.007.
M. Ali, I. Shah, S.W. Kim, M. Sajid, J.H. Lim and K.H. Choi, Quantitative detection of uric acid through ZnO quantum dots based highly sensitive electrochemical biosensor. Sensors and Actuators A: Physical (2018). https://doi.org/10.1016/j.sna.2018.10.009.
S. Jain, S. Verma, S.P. Singh and S.N. Sharma, An electrochemical biosensor based on novel butylamine capped CZTS nanoparticles immobilized by uricase for uric acid detection. Biosensors and Bioelectronics (2019). https://doi.org/10.1016/j.bios.2018.12.008.
T. Del Castillo-Castro, E. Larios-Rodriguez, Z. Molina-Arenas, M.M. Castillo-Ortega and J. Tanori, Synthesis and characterization of metallic nanoparticles and their incorporation into electroconductive polymer composites. Composites Part A: Applied Science and Manufacturing (2007). https://doi.org/10.1016/S0925-4005(02)00045-X.
Y. Zhang, G. Wen, Y. Zhou, S. Shuang, C. Dong and M.M. Choi, Development and analytical application of an uric acid biosensor using an uricase-immobilized eggshell membrane. Biosensors and Bioelectronics (2007). https://doi.org/10.1016/j.bios.2006.08.038.
F. Zhang, X. Wang, S. Ai, Z. Sun, Q. Wan, Z. Zhu and K. Yamamoto, Immobilization of uricase on ZnO nanorods for a reagentless uric acid biosensor. Analytica Chimica Acta (2004). https://doi.org/10.1016/j.aca.2004.05.070.
J. Kan, X. Pan and C. Chen, Polyaniline–uricase biosensor prepared with template process. Biosensors and Bioelectronics (2004). https://doi.org/10.1016/j.bios.2003.12.032.
G. Liu and J. Justin Gooding, An interface comprising molecular wires and poly (ethylene glycol) spacer units self-assembled on carbon electrodes for studies of protein electrochemistry. Langmuir (2006). https://doi.org/10.1021/la0607510.
M.M. Alam, A.M. Asiri, M.T. Uddin, M.A. Islam, M.R. Awual and M.M. Rahman, Detection of uric acid based on doped ZnO/Ag 2 O/Co 3 O 4 nanoparticle loaded glassy carbon electrode. New Journal of Chemistry (2019). https://doi.org/10.1039/C9NJ01287G.
G. Rocchitta, A. Spanu, S. Babudieri, G. Latte, G. Madeddu, G. Galleri and P.A. Serra, Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors (2016). https://doi.org/10.3390/s16060780.
M. Mohammadniaei, C. Park, J. Min, H. Sohn and T. Lee, Fabrication of electrochemical-based bioelectronic device and biosensor composed of biomaterial-nanomaterial hybrid. Biomimetic Medical Materials (2018). https://doi.org/10.1007/978-981-13-0445-3_17.
A.R. Vijayakumar, E. Csöregi, A. Heller and L. Gorton, Alcohol biosensors based on coupled oxidase-peroxidase systems. Analytica Chimica Acta (1996). https://doi.org/10.1016/0003-2670(96)00093-1.
P.E. Erden and E. Kılıç, A review of enzymatic uric acid biosensors based on amperometric detection. Talanta (2013). https://doi.org/10.1016/j.talanta.2013.01.043.
L.V. Bel’skaya, E.A. Sarf and V.K. Kosenok, Age and gender characteristics of the biochemical composition of saliva: correlations with the composition of blood plasma. Journal of oral biology and craniofacial research (2020). https://doi.org/10.1016/j.jobcr.2020.02.004.
J.M. George, A. Antony and B. Mathew, Metal oxide nanoparticles in electrochemical sensing and biosensing: a review. Microchimica Acta (2018). https://doi.org/10.1007/s00604-018-2894-3.
R. Chen, Y. Wang, Y. Liu and J. Li, Selective electrochemical detection of dopamine using nitrogen-doped graphene/manganese monoxide composites. RSC Advances (2015). https://doi.org/10.1039/C5RA14328D.
H. Zhu, A. Sigdel, S. Zhang, D. Su, Z. Xi, Q. Li and S. Sun, Core/shell Au/MnO nanoparticles prepared through controlled oxidation of AuMn as an electrocatalyst for sensitive H2O2 detection. Angewandte Chemie International Edition (2014). https://doi.org/10.1002/anie.201406281.
S. Zhao, D.W. Wang, R. Amal and L. Dai, Carbon based metal free catalysts for key reactions involved in energy conversion and storage. Advanced Materials (2019). https://doi.org/10.1002/adma.201801526.
S.Y. Kishioka and A. Yamada, Kinetic study of the catalytic oxidation of benzyl alcohols by phthalimide-N-oxyl radical electrogenerated in acetonitrile using rotating disk electrode voltammetry. Journal of Electroanalytical Chemistry (2005). https://doi.org/10.1016/j.jelechem.
H. Karimi-Maleh, F. Karimi, M. Alizadeh and A.L. Sanati, Electrochemical sensors, a bright future in the fabrication of portable kits in analytical systems. Chem. Rec., 20 (2020) 682–692. https://doi.org/10.1002/tcr.201900092.
Y. Pan, J. Zuo, Z. Hou, Y. Huang and C. Huang, Preparation of Electrochemical Sensor Based on Zinc Oxide Nanoparticles for Simultaneous Determination of AA, DA, and UA. Front. Chem., 8 (2020) 592538. https://doi.org/10.3389/fchem.2020.592538.
S. Cimitan, S. Albonetti, L. Forni, F. Peri and D. Lazzari, Solvothermal synthesis and properties control of doped ZnO nanoparticles. Journal of Colloid and Interface Science (2009). https://doi.org/10.1016/j.jcis.2008.09.060.
N.S. Rao and M.B. Rao, Structural and optical investigation of ZnO nanopowders synthesized from zinc chloride and zinc nitrate. Am. J. Mater. Sci, 5 (2015) 66–68.
Bharti, J.S. Jangwan, G. Kumar, V. Kumar and A. Kumar. Abatement of organic and inorganic pollutants from drinking water by using commercial and laboratory-synthesized zinc oxide nanoparticles. SN Appl. Sci., 3 (2021) 311. https://doi.org/10.1007/s42452-021-04294-0
A. Umar, M.M. Rahman, M. Vaseem and Y.-B. Hahn. Ultra-sensitive cholesterol biosensor based on low-temperature grown ZnO nanoparticles. Electrochem. Commun., 11(1) (2009) 118–121. https://doi.org/10.1016/j.elecom.2008.10.046
F. Bigdeli, A. Morsali and P. Retailleau, Syntheses and characterization of different zinc (II) oxide nano-structures from direct thermal decomposition of 1D coordination polymers. Polyhedron (2010). https://doi.org/10.1016/j.poly.2009.10.027.
R. Cuscó, E. Alarcón-Lladó, J. Ibanez, L. Artús, J. Jiménez, B. Wang and M.J. Callahan, Temperature dependence of Raman scattering in ZnO. Physical Review B (2007). https://doi.org/10.1103/PhysRevB.75.165202.
U. Pal, J.G. Serrano, P. Santiago, G. Xiong, K.B. Ucer and R.T. Williams, Synthesis and optical properties of ZnO nanostructures with different morphologies. Optical Materials (2006). https://doi.org/10.1016/j.optmat.2006.03.015.
M. Kashif, M.N. Akhtar, N. Nasir and N. Yahya, Versatility of ZnO nanostructures. Carbon and Oxide Nanostructures. Springer, Berlin (2010).
N.G. Mphuthi, A.S. Adekunle, O.E. Fayemi, L.O. Olasunkanmi and E.E. Ebenso, Phthalocyanine doped metal oxide nanoparticles on multiwalled carbon nanotubes platform for the detection of dopamine. Scientific reports (2017). https://doi.org/10.1038/srep43181.
N.M. Nor, K.A. Razak and Z. Lockman, Physical and electrochemical properties of iron oxide nanoparticles-modified electrode for amperometric glucose detection. Electrochimica Acta (2017). https://doi.org/10.1016/j.electacta.2017.07.097.
N. Elgrishi, K.J. Rountree, B.D. McCarthy, E.S. Rountree, T.T. Eisenhart and J.L. Dempsey, A practical beginner’s guide to cyclic voltammetry. Journal of chemical education (2018). https://doi.org/10.1021/acs.jchemed.7b00361.
Q. Yan, N. Zhi, L. Yang, G. Xu, Q. Feng, Q. Zhang and S. Sun, A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode. Scientific reports (2020). https://doi.org/10.1038/s41598-020-67394-8.
T. Ghosh, P. Sarkar and A.P. Turner, A novel third generation uric acid biosensor using uricase electro-activated with ferrocene on a Nafion coated glassy carbon electrode. Bioelectrochemistry (2015). https://doi.org/10.1016/j.bioelechem.2014.11.001.
K.P. Aryal and H.K. Jeong, Carbon nanofiber modified with reduced graphite oxide for detection of ascorbic acid, dopamine, and uric acid. Chemical Physics Letters (2020). https://doi.org/10.1016/j.cplett.2019.136969.
Acknowledgements
We are grateful to the Director, National Physical Laboratory, New Delhi, India, for providing research facilities. Priyanka Dutta is thankful to UGC for providing research fellowship.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approvel
This article does not contain any studies related to human participants or animal performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutta, P., Sharma, V., Bhardwaj, H. et al. Fabrication of Electrochemical Biosensor Using Zinc Oxide Nanoflowers for the Detection of Uric Acid. MAPAN 37, 585–595 (2022). https://doi.org/10.1007/s12647-022-00598-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12647-022-00598-7