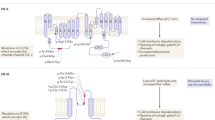
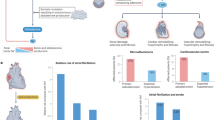
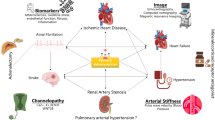
Abstract
Primary aldosteronism is a common cause of hypertension and is a risk factor for cardiovascular and renal morbidity and mortality, via mechanisms mediated by both hypertension and direct insults to target organs. Despite its high prevalence and associated complications, primary aldosteronism remains largely under-recognized, with less than 2% of people in at-risk populations ever tested. Fundamental progress made over the past decade has transformed our understanding of the pathogenesis of primary aldosteronism and of its clinical phenotypes. The dichotomous paradigm of primary aldosteronism diagnosis and subtyping is being redefined into a multidimensional spectrum of disease, which spans subclinical stages to florid primary aldosteronism, and from single-focal or multifocal to diffuse aldosterone-producing areas, which can affect one or both adrenal glands. This Review discusses how redefining the primary aldosteronism syndrome as a multidimensional spectrum will affect the approach to the diagnosis and subtyping of primary aldosteronism.
Key points
-
Primary aldosteronism is a syndrome of dysregulated aldosterone production, independent of renin and other physiological aldosterone regulators.
-
Primary aldosteronism is an independent risk factor for cardiovascular, renal and metabolic morbidity.
-
Although primary aldosteronism is a common cause of hypertension, it is rarely tested for, and testing typically captures severe disease after serious comorbidities have developed.
-
Primary aldosteronism spans a continuum that evolves from subclinical stages to florid disease and includes single-focal or multifocal aldosterone-producing areas that affect one or both adrenal glands.
-
Early detection and targeted therapy of primary aldosteronism could prevent cardiovascular and renal comorbidity and mortality in many patients with hypertension.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Conn, J. W. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J. Lab. Clin. Med. 45, 3–17 (1955).
Wannachalee, T., Lieberman, L. & Turcu, A. F. High prevalence of autonomous aldosterone production in hypertension: how to identify and treat it. Curr. Hypertens. Rep. 24, 123–132 (2022).
Zennaro, M. C., Boulkroun, S. & Fernandes-Rosa, F. L. Pathogenesis and treatment of primary aldosteronism. Nat. Rev. Endocrinol. 16, 578–589 (2020).
Fournier, D., Luft, F. C., Bader, M., Ganten, D. & Andrade-Navarro, M. A. Emergence and evolution of the renin-angiotensin-aldosterone system. J. Mol. Med. 90, 495–508 (2012).
Denton, D. The Hunger for Salt: An Anthropological, Physiological, and Medical Analysis 1st edn (Springer, 1984).
Vaidya, A., Mulatero, P., Baudrand, R. & Adler, G. K. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr. Rev. 39, 1057–1088 (2018).
Kronenberg, H., Melmed, S., Polonsky, K. & Larsen, P. Williams Textbook of Endocrinology 11th edn (Saunders, 2008).
St-Jean, M., Bourdeau, I., Martin, M. & Lacroix, A. Aldosterone is aberrantly regulated by various stimuli in a high proportion of patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 106, e45–e60 (2021).
Zwermann, O., Suttmann, Y., Bidlingmaier, M., Beuschlein, F. & Reincke, M. Screening for membrane hormone receptor expression in primary aldosteronism. Eur. J. Endocrinol. 160, 443–451 (2009).
Palmer, B. F. Regulation of potassium homeostasis. Clin. J. Am. Soc. Nephrol. 10, 1050–1060 (2015).
Arroyo, J. P., Ronzaud, C., Lagnaz, D., Staub, O. & Gamba, G. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology 26, 115–123 (2011).
Redheuil, A. et al. Aldosterone-related myocardial extracellular matrix expansion in hypertension in humans: a proof-of-concept study by cardiac magnetic resonance. JACC Cardiovasc. Imaging 13, 2149–2159 (2020).
Gaddam, K. et al. Rapid reversal of left ventricular hypertrophy and intracardiac volume overload in patients with resistant hypertension and hyperaldosteronism: a prospective clinical study. Hypertension 55, 1137–1142 (2010).
Rocha, R. et al. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology 141, 3871–3878 (2000).
Martinez, D. V. et al. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension 39, 614–618 (2002).
Brilla, C. G. & Weber, K. T. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J. Lab. Clin. Med. 120, 893–901 (1992).
Tesch, G. H. & Young, M. J. Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front. Pharmacol. 8, 313 (2017).
Monticone, S. et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J. Am. Coll. Cardiol. 69, 1811–1820 (2017).
Murata, M. et al. Plasma aldosterone level within the normal range is less associated with cardiovascular and cerebrovascular risk in primary aldosteronism. J. Hypertens. 35, 1079–1085 (2017).
Catena, C. et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch. Intern. Med. 168, 80–85 (2008).
Milliez, P. et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 45, 1243–1248 (2005).
Mulatero, P. et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 98, 4826–4833 (2013).
Takeda, R., Matsubara, T., Miyamori, I., Hatakeyama, H. & Morise, T. Vascular complications in patients with aldosterone producing adenoma in Japan: comparative study with essential hypertension. The Research Committee of Disorders of Adrenal Hormones in Japan. J. Endocrinol. Invest. 18, 370–373 (1995).
Reincke, M. et al. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension 60, 618–624 (2012).
Savard, S., Amar, L., Plouin, P. F. & Steichen, O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension 62, 331–336 (2013).
Hundemer, G. L., Curhan, G. C., Yozamp, N., Wang, M. & Vaidya, A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 6, 51–59 (2018).
Rossi, G. P. et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension 62, 62–69 (2013).
Rossi, G. P. et al. Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension 71, 585–591 (2018).
Fallo, F. et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J. Clin. Endocrinol. Metab. 91, 454–459 (2006).
Chen, W., Li, F., He, C., Zhu, Y. & Tan, W. Elevated prevalence of abnormal glucose metabolism in patients with primary aldosteronism: a meta-analysis. Ir. J. Med. Sci. 183, 283–291 (2014).
Hanslik, G. et al. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn’s Registry. Eur. J. Endocrinol. 173, 665–675 (2015).
Rossi, G. P. et al. Renal damage in primary aldosteronism: results of the PAPY study. Hypertension 48, 232–238 (2006).
Reincke, M. et al. Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J. Clin. Endocrinol. Metab. 94, 869–875 (2009).
Hundemer, G. et al. Renin phenotypes to characterize vascular disease, autonomous aldosteronism, and mineralocorticoid receptor activity. J. Clin. Endocrinol. Metab. 102, 1835–1843 (2017).
Sechi, L. A. et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA 295, 2638–2645 (2006).
Salcuni, A. S. et al. Bone involvement in aldosteronism. J. Bone Miner. Res. 27, 2217–2222 (2012).
Salcuni, A. S. et al. Primary aldosteronism as a cause of secondary osteoporosis. Eur. J. Endocrinol. 177, 431–437 (2017).
Ceccoli, L. et al. Bone health and aldosterone excess. Osteoporos. Int. 24, 2801–2807 (2013).
Wu, V. C. et al. Risk of fracture in primary aldosteronism: a population-based cohort study. J. Bone Miner. Res. 32, 743–752 (2017).
Velema, M. S. et al. Health-related quality of life and mental health in primary aldosteronism: a systematic review. Horm. Metab. Res. 49, 943–950 (2017).
Buffolo, F. et al. Quality of life in primary aldosteronism: a prospective observational study. Eur. J. Clin. Invest. 51, e13419 (2021).
Saha, S., Bornstein, S. R., Graessler, J., Chakrabarti, S. & Kopprasch, S. Aldosterone hypothesis for cognitive impairment in diabetes mellitus. Horm. Metab. Res. 49, 716–718 (2017).
Gomez-Sanchez, E. P. Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis. Steroids 91, 20–31 (2014).
Horiuchi, M. Brain renin-angiotensin-aldosterone system and cognitive function [Japanese]. Nihon Rinsho 72, 641–647 (2014).
Loprinzi, P. D. & Frith, E. Obesity and episodic memory function. J. Physiol. Sci. 68, 321–331 (2018).
de Kloet, E. R., Meijer, O. C., de Nicola, A. F., de Rijk, R. H. & Joels, M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocrinol. 49, 124–145 (2018).
Rotenstein, L. S., Sheridan, M., Garg, R. & Adler, G. K. Effect of mineralocorticoid receptor blockade on hippocampal-dependent memory in adults with obesity. Obesity 23, 1136–1142 (2015).
Monticone, S. et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 6, 41–50 (2018).
Prete, A. et al. Cardiometabolic disease burden and steroid excretion in benign adrenal tumors: a cross-sectional multicenter study. Ann. Intern. Med. 175, 325–334 (2022).
Bancos, I. & Prete, A. Approach to the patient with adrenal incidentaloma. J. Clin. Endocrinol. Metab. 106, 3331–3353 (2021).
Vaidya, A., Hamrahian, A., Bancos, I., Fleseriu, M. & Ghayee, H. K. The evaluation of incidentally discovered adrenal masses. Endocr. Pract. 25, 178–192 (2019).
Arlt, W. et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight 2, e93136 (2017).
Gerards, J. et al. Impaired glucose metabolism in primary aldosteronism is associated with cortisol cosecretion. J. Clin. Endocrinol. Metab. 104, 3192–3202 (2019).
Adolf, C. et al. Cortisol excess in patients with primary aldosteronism impacts left ventricular hypertrophy. J. Clin. Endocrinol. Metab. 103, 4543–4552 (2018).
Zelinka, T. et al. Postoperative adrenal insufficiency in Conn’s syndrome – does it occur frequently? J. Hum. Hypertens. 36, 510–516 (2022).
DeLozier, O. M. et al. Selective glucocorticoid replacement following unilateral adrenalectomy for hypercortisolism and primary aldosteronism. J. Clin. Endocrinol. Metab. 107, e538–e547 (2022).
Heinrich, D. A. et al. Adrenal insufficiency after unilateral adrenalectomy in primary aldosteronism: long-term outcome and clinical impact. J. Clin. Endocrinol. Metab. 104, 5658–5664 (2019).
Fassnacht, M. et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 175, G1–G34 (2016).
Zennaro, M. C., Boulkroun, S. & Fernandes-Rosa, F. Genetic causes of functional adrenocortical adenomas. Endocr. Rev. 38, 516–537 (2017).
Gomez-Sanchez, C. E. et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol. Cell. Endocrinol. 383, 111–117 (2014).
Nanba, K. et al. Genetic characteristics of aldosterone-producing adenomas in Blacks. Hypertension 73, 885–892 (2019).
Nanba, K., Vaidya, A. & Rainey, W. E. Aging and adrenal aldosterone production. Hypertension 71, 218–223 (2018).
Nanba, K. et al. Age-related autonomous aldosteronism. Circulation 136, 347–355 (2017).
Nishimoto, K. et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc. Natl Acad. Sci. USA 112, E4591–E4599 (2015).
Omata, K. et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J. Endocr. Soc. 1, 787–799 (2017).
Omata, K., Tomlins, S. A. & Rainey, W. E. Aldosterone-producing cell clusters in normal and pathological states. Horm. Metab. Res. 49, 951–956 (2017).
Omata, K. et al. Genetic and histopathologic intertumor heterogeneity in primary aldosteronism. J. Clin. Endocrinol. Metab. 102, 1792–1796 (2017).
Williams, T. A. & Reincke, M. Pathophysiology and histopathology of primary aldosteronism. Trends Endocrinol. Metab. 33, 36–49 (2022).
Baudrand, R. et al. Continuum of renin-independent aldosteronism in normotension. Hypertension 69, 950–956 (2017).
Brown, J. M. et al. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann. Intern. Med. 167, 630–641 (2017).
Brown, J. M. et al. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension 63, 1205–1211 (2014).
Markou, A. et al. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: a very high odds ratio for progression into arterial hypertension. J. Clin. Endocrinol. Metab. 98, 1409–1416 (2013).
Vaidya, A. et al. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension 61, 886–893 (2013).
Brown, J. M. et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann. Intern. Med. 173, 10–20 (2020).
Vasan, R. S. et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N. Engl. J. Med. 351, 33–41 (2004).
Bentley-Lewis, R. et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J. Clin. Endocrinol. Metab. 92, 4472–4475 (2007).
Brown, J. M. et al. Cardiac structure and function across the spectrum of aldosteronism: the atherosclerosis risk in communities study. Hypertension https://doi.org/10.1161/HYPERTENSIONAHA.122.19134 (2022).
Inoue, K. et al. Serum aldosterone concentration, blood pressure, and coronary artery calcium: the multi-ethnic study of atherosclerosis. Hypertension 76, 113–120 (2020).
Hu, J. et al. Heightened cardiovascular risk in hypertension associated with renin-independent aldosteronism versus renin-dependent aldosteronism: a collaborative study. J. Am. Heart Assoc. 10, e023082 (2021).
Joseph, J. J. et al. Association of serum aldosterone and plasma renin activity with ambulatory blood pressure in African Americans: the Jackson Heart Study. Circulation 143, 2355–2366 (2021).
Adlin, E. V. Letter: Plasma-renin and blood-pressure. Lancet 1, 699 (1975).
Newton-Cheh, C. et al. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension 49, 846–856 (2007).
Williams, B. et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 386, 2059–2068 (2015).
Williams, B. et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 6, 464–475 (2018).
Carey, R. M., Douglas, J. G., Schweikert, J. R. & Liddle, G. W. The syndrome of essential hypertension and suppressed plasma renin activity. Normalization of blood pressure with spironolactone. Arch. Intern. Med. 130, 849–854 (1972).
Käyser, S. C. et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J. Clin. Endocrinol. Metab. 101, 2826–2835 (2016).
Xu, Z. et al. Primary aldosteronism in patients in China with recently detected hypertension. J. Am. Coll. Cardiol. 75, 1913–1922 (2020).
Libianto, R. et al. Detecting primary aldosteronism in Australian primary care: a prospective study. Med. J. Aust. 216, 408–412 (2022).
Voulgaris, N. et al. Prevalence of primary aldosteronism across the stages of hypertension based on a new combined overnight test. Horm. Metab. Res. 53, 461–469 (2021).
Alam, S. et al. High prevalence and a long delay in the diagnosis of primary aldosteronism among patients with young-onset hypertension. Clin. Endocrinol. 94, 895–903 (2021).
Funder, J. W. et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 101, 1889–1916 (2016).
Carey, R. M. et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension 72, e53–e90 (2018).
Buffolo, F. et al. Primary aldosteronism and obstructive sleep apnea: a cross-sectional multi-ethnic study. Hypertension 74, 1532–1540 (2019).
Rossi, G. P. & Dalla Cà, A. Clinical management of primary aldosteronism: 2013 practical recommendations of the Italian Society of Hypertension (SIIA). High. Blood Press. Cardiovasc. Prev. 21, 71–75 (2014).
Amar, L. et al. SFE/SFHTA/AFCE primary aldosteronism consensus: introduction and handbook. Ann. Endocrinol. 77, 179–186 (2016).
Gkaniatsa, E. et al. Increasing incidence of primary aldosteronism in western Sweden during 3 decades–yet an underdiagnosed disorder. J. Clin. Endocrinol. Metab. 106, e3603–e3610 (2021).
Sivarajah, M., Beninato, T. & Fahey, T. J. III Adherence to consensus guidelines for screening of primary aldosteronism in an urban healthcare system. Surgery 167, 211–215 (2020).
Liu, Y.-Y. et al. Outcomes of a specialized clinic on rates of investigation and treatment of primary aldosteronism. JAMA Surg. 156, 541–549 (2021).
Mulatero, P. et al. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J. Hypertens. 34, 2253–2257 (2016).
Yang, J., Fuller, P. J. & Stowasser, M. Is it time to screen all patients with hypertension for primary aldosteronism? Med. J. Aust. 209, 57–59 (2018).
Burrello, J. et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension 75, 1025–1033 (2020).
Saito, K. et al. Subtype-specific trends in the clinical picture of primary aldosteronism over a 13-year period. J. Hypertens. 39, 2325–2332 (2021).
Hundemer, G. L. et al. Screening rates for primary aldosteronism among individuals with hypertension plus hypokalemia: a population-based retrospective cohort study. Hypertension 79, 178–186 (2022).
Jaffe, G. et al. Screening rates for primary aldosteronism in resistant hypertension. Hypertension 75, 650–659 (2020).
Cohen, J. B. et al. Testing for primary aldosteronism and mineralocorticoid receptor antagonist use among US veterans: a retrospective cohort study. Ann. Intern. Med. 174, 289–297 (2021).
Lim, Y. Y. et al. Impact of Victoria’s first dedicated endocrine hypertension service on the pattern of primary aldosteronism diagnoses. Intern. Med. J. 51, 1255–1261 (2021).
Chauhan, K. et al. Screening for primary aldosteronism is underutilised in patients with chronic kidney disease. J. Nephrol. https://doi.org/10.1007/s40620-022-01267-3 (2022).
Tan, S. J., Libianto, R., Yang, J. & Wong, J. Screening for primary aldosteronism in the diabetic population: a cohort study. Intern. Med. J. https://doi.org/10.1111/imj.15690 (2022).
Turcu, A. F. et al. Primary aldosteronism screening rates differ with sex, race, and comorbidities. J. Am. Heart Assoc. 11, e025952 (2022).
Pitt, B. et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl. J. Med. 385, 2252–2263 (2021).
Filippatos, G. et al. Finerenone reduces new-onset atrial fibrillation in patients with chronic kidney disease and type 2 diabetes. J. Am. Coll. Cardiol. 78, 142–152 (2021).
Filippatos, G. et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation 143, 540–552 (2021).
Filippatos, G. et al. Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes: analyses from the FIGARO-DKD trial. Circulation 145, 437–447 (2022).
Chen, Y. et al. Strain imaging for the early detection of cardiac remodeling and dysfunction in primary aldosteronism. Diagnostics 12, 543 (2022).
Stowasser, M. in Encyclopedia of Endocrine Diseases 2nd edn (eds Huhtaniemi, I. & Martini, L.) 598–606 (Academic, 2019).
Nishikawa, T. et al. Guidelines for the diagnosis and treatment of primary aldosteronism — the Japan Endocrine Society 2009. Endocr. J. 58, 711–721 (2011).
Li, N. et al. Cost-effectiveness analysis of screening for primary aldosteronism in China. Clin. Endocrinol. 95, 414–422 (2021).
Buffolo, F. et al. Clinical score and machine learning-based model to predict diagnosis of primary aldosteronism in arterial hypertension. Hypertension 78, 1595–1604 (2021).
Burrello, J. et al. Development and validation of prediction models for subtype diagnosis of patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 105, dgaa379 (2020).
Kocjan, T., Janez, A., Stankovic, M., Vidmar, G. & Jensterle, M. A new clinical prediction criterion accurately determines a subset of patients with bilateral primary aldosteronism before adrenal venous sampling. Endocr. Pract. 22, 587–594 (2016).
Zhang, Y. et al. Identifying unilateral disease in Chinese patients with primary aldosteronism by using a modified prediction score. J. Hypertens. 35, 2486–2492 (2017).
Sam, D. et al. External validation of clinical prediction models in unilateral primary aldosteronism. Am. J. Hypertens. 35, 365–373 (2022).
Hiramatsu, K. et al. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity. Results in hypertensive patients. Arch. Intern. Med. 141, 1589–1593 (1981).
Yozamp, N. et al. Intraindividual variability of aldosterone concentrations in primary aldosteronism. Hypertension 77, 891–899 (2021).
Galati, S.-J. et al. Prevelence of primary aldosteronism in an urban hypertensive population. Endocr. Pract. 22, 1296–1302 (2016).
Stowasser, M. et al. High rate of detection of primary aldosteronism, including surgically treatable forms, after ‘non-selective’ screening of hypertensive patients. J. Hypertens. 21, 2149–2157 (2003).
Stowasser, M. & Gordon, R. D. Aldosterone assays: an urgent need for improvement. Clin. Chem. 52, 1640–1642 (2006).
Guo, Z. et al. Aldosterone LC-MS/MS assay-specific threshold values in screening and confirmatory testing for primary aldosteronism. J. Clin. Endocrinol. Metab. 103, 3965–3973 (2018).
Mulatero, P. et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension 40, 897–902 (2002).
Tezuka, Y. & Turcu, A. F. Mineralocorticoid receptor antagonists decrease the rates of positive screening for primary aldosteronism. Endocr. Pract. 26, 1416–1424 (2020).
Pecori, A. et al. Mineralocorticoid receptor antagonist effect on aldosterone to renin ratio in patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 106, e3655–e3664 (2021).
Rossi, G. P. et al. Effects of mineralocorticoid and AT1 receptor antagonism on the aldosterone-renin ratio in primary aldosteronism–the EMIRA study. J. Clin. Endocrinol. Metab. 105, dgaa080 (2020).
Nanba, A. T. et al. Adrenal vein sampling lateralization despite mineralocorticoid receptor antagonists exposure in primary aldosteronism. J. Clin. Endocrinol. Metab. 104, 487–492 (2019).
Hundemer, G. L., Kline, G. A. & Leung, A. A. How common is primary aldosteronism? Curr. Opin. Nephrol. Hypertens. 30, 353–360 (2021).
Li, X., Goswami, R., Yang, S. & Li, Q. Aldosterone/direct renin concentration ratio as a screening test for primary aldosteronism: a meta-analysis. J. Renin Angiotensin Aldosterone Syst. 17, 1470320316657450 (2016).
Jansen, P. M. et al. Test characteristics of the aldosterone-to-renin ratio as a screening test for primary aldosteronism. J. Hypertens. 32, 115–126 (2014).
Hung, A. et al. Performance of the aldosterone to renin ratio as a screening test for primary aldosteronism. J. Clin. Endocrinol. Metab. 106, 2423–2435 (2021).
Funder, J. W. Primary aldosteronism: at the tipping point. Ann. Intern. Med. 173, 65–66 (2020).
[No authors listed] Renin suppression in primary aldosteronism. JAMA 207, 747 (1969).
Oelkers, W., Diederich, S. & Bähr, V. Primary hyperaldosteronism without suppressed renin due to secondary hypertensive kidney damage. J. Clin. Endocrinol. Metab. 85, 3266–3270 (2000).
Jansen, P. M. & Stowasser, M. Aldosterone-producing adenoma associated with non-suppressed renin: a case series. J. Hum. Hypertens. 36, 373–380 (2022).
Wang, K. et al. Development and validation of criteria for sparing confirmatory tests in diagnosing primary aldosteronism. J. Clin. Endocrinol. Metab. 105, dgaa282 (2020).
Umakoshi, H. et al. Role of aldosterone and potassium levels in sparing confirmatory tests in primary aldosteronism. J. Clin. Endocrinol. Metab. 105, dgz148 (2020).
Vaidya, A. & Carey, R. M. Evolution of the primary aldosteronism syndrome: updating the approach. J. Clin. Endocrinol. Metab. 105, 3771–3783 (2020).
Lim, P. O., Jung, R. T. & MacDonald, T. M. Raised aldosterone to renin ratio predicts antihypertensive efficacy of spironolactone: a prospective cohort follow-up study. Br. J. Clin. Pharmacol. 48, 756–760 (1999).
Leung, A. A. et al. Performance of confirmatory tests for diagnosing primary aldosteronism: a systematic review and meta-analysis. Hypertension https://doi.org/10.1161/HYPERTENSIONAHA.122.19377 (2022).
Wu, S. et al. Confirmatory tests for the diagnosis of primary aldosteronism: a systematic review and meta-analysis. Clin. Endocrinol. 90, 641–648 (2019).
Rossi, G. P. et al. Comparison of the captopril and the saline infusion test for excluding aldosterone-producing adenoma. Hypertension 50, 424–431 (2007).
Song, Y. et al. Confirmatory tests for the diagnosis of primary aldosteronism: a prospective diagnostic accuracy study. Hypertension 71, 118–124 (2018).
Stowasser, M. et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J. Clin. Endocrinol. Metab. 103, 4113–4124 (2018).
Thuzar, M. et al. Diagnosis of primary aldosteronism by seated saline suppression test-variability between immunoassay and HPLC-MS/MS. J. Clin. Endocrinol. Metab. 105, dgz150 (2020).
Cornu, E. et al. Suppression of aldosterone secretion after recumbent saline infusion does not exclude lateralized primary aldosteronism. Hypertension 68, 989–994 (2016).
Eisenhofer, G. et al. The saline infusion test for primary aldosteronism: implications of immunoassay inaccuracy. J. Clin. Endocrinol. Metab. 107, e2027–e2036 (2022).
Liu, B. et al. Seated saline suppression test is comparable with captopril challenge test for the diagnosis of primary aldosteronism: a prospective study. Endocr. Pract. 27, 326–333 (2021).
Markou, A. et al. Enhanced performance of a modified diagnostic test of primary aldosteronism in patients with adrenal adenomas. Eur. J. Endocrinol. 186, 265–273 (2022).
Williams, T. A. et al. International histopathology consensus for unilateral primary aldosteronism. J. Clin. Endocrinol. Metab. 106, 42–54 (2021).
Nanba, A. T. et al. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin. Endocrinol. 87, 665–672 (2017).
Yamazaki, Y. et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J. Clin. Endocrinol. Metab. 102, 1182–1192 (2017).
Omata, K. et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension 72, 874–880 (2018).
Nanba, K. & Rainey, W. E. Pathophysiology of bilateral hyperaldosteronism. Curr. Opin. Endocrinol. Diabetes Obes. 29, 233–242 (2022).
Hacini, I. et al. Somatic mutations in adrenals from patients with primary aldosteronism not cured after adrenalectomy suggest common pathogenic mechanisms between unilateral and bilateral disease. Eur. J. Endocrinol. 185, 405–412 (2021).
De Sousa, K. et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension 75, 1034–1044 (2020).
Nanba, K. et al. Double adrenocortical adenomas harboring independent KCNJ5 and PRKACA somatic mutations. Eur. J. Endocrinol. 175, K1–K6 (2016).
Dekkers, T. et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J. Clin. Endocrinol. Metab. 99, E1341–E1351 (2014).
Williams, T. A. et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 5, 689–699 (2017).
Hundemer, G. L., Curhan, G. C., Yozamp, N., Wang, M. & Vaidya, A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 3, 768–774 (2018).
Hundemer, G. L., Curhan, G. C., Yozamp, N., Wang, M. & Vaidya, A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension 72, 658–666 (2018).
Ebbehoj, A. et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 8, 894–902 (2020).
Umakoshi, H. et al. Accuracy of adrenal computed tomography in predicting the unilateral subtype in young patients with hypokalaemia and elevation of aldosterone in primary aldosteronism. Clin. Endocrinol. 88, 645–651 (2018).
Lim, V. et al. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. J. Clin. Endocrinol. Metab. 99, 2712–2719 (2014).
Wannachalee, T. et al. The concordance between imaging and adrenal vein sampling varies with aldosterone-driver somatic mutation. J. Clin. Endocrinol. Metab. 105, e3628–e3637 (2020).
Rossi, G. P. et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 63, 151–160 (2014).
Naruse, M. et al. Japan Endocrine Society clinical practice guideline for the diagnosis and management of primary aldosteronism 2021. Endocr. J. 69, 327–359 (2022).
Mulatero, P. et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 38, 1929–1936 (2020).
Lethielleux, G., Amar, L., Raynaud, A., Plouin, P. F. & Steichen, O. Influence of diagnostic criteria on the interpretation of adrenal vein sampling. Hypertension 65, 849–854 (2015).
El Ghorayeb, N. et al. Basal and post-ACTH aldosterone and its ratios are useful during adrenal vein sampling in primary aldosteronism. J. Clin. Endocrinol. Metab. 101, 1826–1835 (2016).
Desrochers, M. J. et al. Basal contralateral aldosterone suppression is rare in lateralized primary aldosteronism. Eur. J. Endocrinol. 183, 399–409 (2020).
Dominguez, D. A. et al. Contralateral suppression index does not predict clinical cure in patients undergoing surgery for primary aldosteronism. Ann. Surg. Oncol. 28, 7487–7495 (2021).
Monticone, S. et al. Aldosterone suppression on contralateral adrenal during adrenal vein sampling does not predict blood pressure response after adrenalectomy. J. Clin. Endocrinol. Metab. 99, 4158–4166 (2014).
Shibayama, Y. et al. Bilateral aldosterone suppression and its resolution in adrenal vein sampling of patients with primary aldosteronism: analysis of data from the WAVES-J study. Clin. Endocrinol. 85, 696–702 (2016).
Tan, S. Y. T. et al. Bilateral aldosterone suppression in patients with right unilateral primary aldosteronism and review of the literature. J. Endocr. Soc. 4, bvaa033 (2020).
Kitamoto, T. et al. Precise mapping of intra-adrenal aldosterone activities provides a novel surgical strategy for primary aldosteronism. Hypertension 76, 976–984 (2020).
Rossi, G. P. et al. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J. Hypertension 26, 989–997 (2008).
Seccia, T. M. et al. Adrenocorticotropic hormone stimulation during adrenal vein sampling for identifying surgically curable subtypes of primary aldosteronism: comparison of 3 different protocols. Hypertension 53, 761–766 (2009).
Rossi, G. P. et al. Dynamic testing with high-dose adrenocorticotrophic hormone does not improve lateralization of aldosterone oversecretion in primary aldosteronism patients. J. Hypertens. 24, 371–379 (2006).
Takeda, Y. et al. Impact of adrenocorticotropic hormone stimulation during adrenal venous sampling on outcomes of primary aldosteronism. J. Hypertens. 37, 1077–1082 (2018).
Chee, N. Y. N. et al. Utility of adrenocorticotropic hormone in adrenal vein sampling despite the occurrence of discordant lateralization. Clin. Endocrinol. 93, 394–403 (2020).
El Ghorayeb, N., Bourdeau, I. & Lacroix, A. Role of ACTH and other hormones in the regulation of aldosterone production in primary aldosteronism. Front. Endocrinol. 7, 72 (2016).
Lim, J. S., Plaska, S. W., Rege, J., Rainey, W. E. & Turcu, A. F. Aldosterone-regulating receptors and aldosterone-driver somatic mutations. Front. Endocrinol. 12, 644382 (2021).
Wolley, M. J., Ahmed, A. H., Gordon, R. D. & Stowasser, M. Does ACTH improve the diagnostic performance of adrenal vein sampling for subtyping primary aldosteronism? Clin. Endocrinol. 85, 703–709 (2016).
Young, W. F. et al. Role for adrenal venous sampling in primary aldosteronism. Surgery 136, 1227–1235 (2004).
Young, W. F. & Stanson, A. W. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin. Endocrinol. 70, 14–17 (2009).
Wannachalee, T. et al. Three discrete patterns of primary aldosteronism lateralization in response to cosyntropin during adrenal vein sampling. J. Clin. Endocrinol. Metab. 104, 5867–5876 (2019).
Rossi, G. P. et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J. Clin. Endocrinol. Metab. 97, 1606–1614 (2012).
Turcu, A. F. & Auchus, R. Approach to the patient with primary aldosteronism: utility and limitations of adrenal vein sampling. J. Clin. Endocrinol. Metab. 106, 1195–1208 (2021).
Almarzooqi, M. K. et al. Adrenal vein sampling in primary aldosteronism: concordance of simultaneous vs sequential sampling. Eur. J. Endocrinol. 176, 159–167 (2017).
Seccia, T. M. et al. A stress reaction affects assessment of selectivity of adrenal venous sampling and of lateralization of aldosterone excess in primary aldosteronism. Eur. J. Endocrinol. 166, 869–875 (2012).
Rossi, G. P. et al. Identification of the etiology of primary aldosteronism with adrenal vein sampling in patients with equivocal computed tomography and magnetic resonance findings: results in 104 consecutive cases. J. Clin. Endocrinol. Metab. 86, 1083–1090 (2001).
Monticone, S. et al. Adrenal vein sampling in primary aldosteronism: towards a standardised protocol. Lancet Diabetes Endocrinol. 3, 296–303 (2015).
Rossi, G. P. et al. Clinical outcomes of 1625 patients with primary aldosteronism subtyped with adrenal vein sampling. Hypertension 74, 800–808 (2019).
Umakoshi, H. et al. Correlation between lateralization index of adrenal venous sampling and standardized outcome in primary aldosteronism. J. Endocr. Soc. 2, 893–902 (2018).
Peng, K. Y. et al. Presence of subclinical hypercortisolism in clinical aldosterone-producing adenomas predicts lower clinical success. Hypertension 76, 1537–1544 (2020).
Fallo, F. et al. Histopathological and genetic characterization of aldosterone-producing adenomas with concurrent subclinical cortisol hypersecretion: a case series. Endocrine 58, 503–512 (2017).
Kline, G. A. et al. Apparent failed and discordant adrenal vein sampling: a potential confounding role of cortisol cosecretion? Clin. Endocrinol. 96, 123–131 (2022).
O’Toole, S. M. et al. Low grade cortisol co-secretion has limited impact on ACTH-stimulated AVS parameters in primary aldosteronism. J. Clin. Endocrinol. Metab. 105, dgaa519 (2020).
Dekkers, T. et al. Plasma metanephrine for assessing the selectivity of adrenal venous sampling. Hypertension 62, 1152–1157 (2013).
Eisenhofer, G. et al. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin. Chem. 62, 514–524 (2016).
Li, H. et al. Adrenal androgen measurement for assessing the selectivity of adrenal venous sampling in primary aldosteronism. Steroids 134, 16–21 (2018).
Turcu, A. F. et al. Comprehensive analysis of steroid biomarkers for guiding primary aldosteronism subtyping. Hypertension 75, 183–192 (2020).
Zhou, Y. et al. Diagnostic accuracy of adrenal imaging for subtype diagnosis in primary aldosteronism: systematic review and meta-analysis. BMJ Open 10, e038489 (2020).
Dekkers, T. et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 4, 739–746 (2016).
Rossi, G. P. & Funder, J. W. Adrenal venous sampling versus computed tomographic scan to determine treatment in primary aldosteronism (the SPARTACUS trial): a critique. Hypertension 69, 396–397 (2017).
Beuschlein, F. et al. The SPARTACUS trial: controversies and unresolved issues. Horm. Metab. Res. 49, 936–942 (2017).
Williams, T. A. et al. Computed tomography and adrenal venous sampling in the diagnosis of unilateral primary aldosteronism. Hypertension 72, 641–649 (2018).
Stowasser, M., Bachmann, A. W., Tunny, T. J. & Gordon, R. D. Production of 18-oxo-cortisol in subtypes of primary aldosteronism. Clin. Exp. Pharmacol. Physiol. 23, 591–593 (1996).
Mosso, L., Gomez-Sanchez, C. E., Foecking, M. F. & Fardella, C. Serum 18-hydroxycortisol in primary aldosteronism, hypertension, and normotensives. Hypertension 38, 688–691 (2001).
Gordon, R. D., Hamlet, S. M., Tunny, T. J., Gomez-Sanchez, C. E. & Jayasinghe, L. S. Distinguishing aldosterone-producing adenoma from other forms of hyperaldosteronism and lateralizing the tumour pre-operatively. Clin. Exp. Pharmacol. Physiol. 13, 325–328 (1986).
Chu, M. D. & Ulick, S. Isolation and identification of 18-hydroxycortisol from the urine of patients with primary aldosteronism. J. Biol. Chem. 257, 2218–2224 (1982).
Ulick, S., Blumenfeld, J. D., Atlas, S. A., Wang, J. Z. & Vaughan, E. D. Jr The unique steroidogenesis of the aldosteronoma in the differential diagnosis of primary aldosteronism. J. Clin. Endocrinol. Metab. 76, 873–878 (1993).
Hamlet, S. M., Gordon, R. D., Gomez-Sanchez, C. E., Tunny, T. J. & Klemm, S. A. Adrenal transitional zone steroids, 18-oxo and 18-hydroxycortisol, useful in the diagnosis of primary aldosteronism, are ACTH-dependent. Clin. Exp. Pharmacol. Physiol. 15, 317–322 (1988).
Gomez-Sanchez, C. E. et al. Elevated urinary excretion of 18-oxocortisol in glucocorticoid-suppressible aldosteronism. J. Clin. Endocrinol. Metab. 59, 1022–1024 (1984).
Satoh, F. et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension 65, 1096–1102 (2015).
Zheng, F. F. et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension 65, 622–628 (2015).
Taguchi, R. et al. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J. Clin. Endocrinol. Metab. 97, 1311–1319 (2012).
Akerstrom, T. et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS ONE 7, e41926 (2012).
Boulkroun, S. et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension 59, 592–598 (2012).
Azizan, E. A. et al. Somatic mutations affecting the selectivity filter of KCNJ5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension 59, 587–591 (2012).
Monticone, S. et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J. Clin. Endocrinol. Metab. 97, E1567–E1572 (2012).
Nanba, K. et al. Targeted molecular characterization of aldosterone-producing adenomas in white Americans. J. Clin. Endocrinol. Metab. 103, 3869–3876 (2018).
Azizan, E. A. et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J. Clin. Endocrinol. Metab. 97, E819–E829 (2012).
Tezuka, Y. et al. 18-Oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension 73, 1283–1290 (2019).
Nakamura, Y. et al. Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double- and triple-immunostaining for CYP11B2/B1 and CYP17. Mol. Cell Endocrinol. 422, 57–63 (2016).
Eisenhofer, G. et al. Use of steroid profiling combined with machine learning for identification and subtype classification in primary aldosteronism. JAMA Netw. Open 3, e2016209 (2020).
Wong, K. K., Komissarova, M., Avram, A. M., Fig, L. M. & Gross, M. D. Adrenal cortical imaging with I-131 NP-59 SPECT-CT. Clin. Nucl. Med. 35, 865–869 (2010).
Burton, T. J. et al. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J. Clin. Endocrinol. Metab. 97, 100–109 (2012).
Heinze, B. et al. Targeting CXCR4 (CXC chemokine receptor type 4) for molecular imaging of aldosterone-producing adenoma. Hypertension 71, 317–325 (2018).
Sander, K. et al. Development of [(18)F]AldoView as the first highly selective aldosterone synthase PET tracer for imaging of primary hyperaldosteronism. J. Med. Chem. 64, 9321–9329 (2021).
Kaneko, H. et al. Machine learning based models for prediction of subtype diagnosis of primary aldosteronism using blood test. Sci. Rep. 11, 9140 (2021).
Kobayashi, H. et al. Development and validation of subtype prediction scores for the workup of primary aldosteronism. J. Hypertens. 36, 2269–2276 (2018).
Nagano, H. et al. Aldosterone reduction rate after saline infusion test may be a novel prediction in patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 105, dgz092 (2020).
Umakoshi, H. et al. Significance of computed tomography and serum potassium in predicting subtype diagnosis of primary aldosteronism. J. Clin. Endocrinol. Metab. 103, 900–908 (2018).
Hashimura, H. et al. Saline suppression test parameters may predict bilateral subtypes of primary aldosteronism. Clin. Endocrinol. 89, 308–313 (2018).
Acknowledgements
A.F.T. was supported by grants 2019087 from the Doris Duke Charitable Foundation, R01HL155834 from the National Heart, Lung, and Blood Institute, and UL1TR002240 from the Michigan Institute for Clinical and Health Research. A.V. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases under award R01 DK115392-04 and the National Institute of Heart, Lung, and Blood Disorders under award R01 HL153004 and by the Brigham and Women’s Hospital Department of Medicine Innovation Evergreen Fund Award. J.Y. was supported by the National Health and Medical Research Council Investigator Grant APP1194576.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
A.F.T. has received consulting fees from CinCor Pharma and PhaseBio. A.V. reports consulting activities with Mineralys Therapeutics, Corcept Therapeutics, and HRA Pharma, all unrelated to the current work. J.Y. declares no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Jaap Deinum and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turcu, A.F., Yang, J. & Vaidya, A. Primary aldosteronism — a multidimensional syndrome. Nat Rev Endocrinol 18, 665–682 (2022). https://doi.org/10.1038/s41574-022-00730-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-022-00730-2
This article is cited by
-
Simple standing test without furosemide is useful in the diagnosis of primary aldosteronism
Scientific Reports (2023)
-
Epidemiology and diagnosis of primary aldosteronism. What have we learned from the SPAIN-ALDO registry?
Endocrine (2023)
-
Advances in the molecular imaging of primary aldosteronism
Annals of Nuclear Medicine (2023)