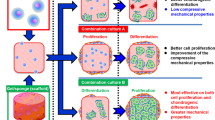
Abstract
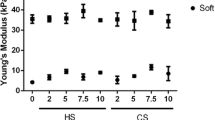
Articular cartilage is one of the most important weight-bearing components in human body, thus the chondrogenesis of stem cells is reactive to many intracellular and extracellular mechanical signals. As a unique physical cue, matrix stiffness plays an integral role in commitment of stem cell fate. However, when examining the downstream effects of matrix stiffness, most studies used different soluble factors to assist physical inducing process, which may mask the chondrogenic effects of matrix stiffness. Here we fabricated polyacrylamide (PAAm) hydrogels with gradient stiffness to unravel the role of matrix stiffness in chondrogenic process of mesenchymal stem cells (MSCs), with or without TGF-β3 as induction factor. The results showed that with micromass culture mimicking relatively high cell density in vivo, the chondrogenic differentiation of MSCs can be promoted by soft substrates (about 0.5 kPa) independently with assembled cytoskeleton. Further analysis indicated that addition of TGF-β3 generally increased expression level of cartilage-related markers and masked the stiffness-derived expression pattern of hypertrophic markers. These results demonstrate how mechanical cues experienced in developmental context regulate commitment of stem cell fate and have significant impact on the design of tissue regeneration materials.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ahrens PB, Solursh M, Reiter RS (1977) Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol 60(1):69–82
Andreu I, Falcones B, Hurst S, Chahare N, Roca-Cusachs P (2021) The force loading rate drives cell mechanosensing through both reinforcement and fluidization. Nat Commun 12(1):4229
Bashirzadeh Y, Chatterji S, Palmer D, Dumbali S, Qian S, Maruthamuthu V (2018) Stiffness Measurement of Soft Silicone Substrates for Mechanobiology Studies Using a Widefield Fluorescence Microscope. J visualized experiments: JoVE 137. https://doi.org/10.3791/57797
Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA (2013a) The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 34(2):413–421
Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA (2013b) The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 34(2):413–421. https://doi.org/10.1016/j.biomaterials.2012.09.052
Bonyadi Rad E, Musumeci G (2017) Runx2 mediated Induction of Novel Targets ST2 and Runx3 Leads to Cooperative Regulation of Hypertrophic Differentiation in ATDC5 Chondrocytes. Sci Rep 7(1):17947. https://doi.org/10.1038/s41598-017-18044-z
Brielle S, Bavli D, Motzik A, Kan-Tor Y, Sun X, Kozulin C (2021) Delineating the heterogeneity of matrix-directed differentiation toward soft and stiff tissue lineages via single-cell profiling. 118. https://doi.org/10.1073/pnas.2016322118. 19
Čamernik K, Mihelič A, Mihalič R, Haring G, Herman S, Marolt Presen D, Janež A, Trebše R, Marc J, Zupan J (2020) Comprehensive analysis of skeletal muscle- and bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell Res Ther 11(1):146. https://doi.org/10.1186/s13287-020-01657-z
Diederichs S, Klampfleuthner FA, Moradi B, Richter W (2019a) Chondral differentiation of induced pluripotent stem cells without progression into the endochondral pathway. Front cell Dev biology 7:270
Diederichs S, Tonnier V, März M, Dreher SI, Geisbüsch A, Richter W (2019b) Regulation of WNT5A and WNT11 during MSC in vitro chondrogenesis: WNT inhibition lowers BMP and hedgehog activity, and reduces hypertrophy. Cell Mol Life Sci 76(19):3875–3889
Fischer RS, Myers KA, Gardel ML, Waterman CM (2012) Stiffness-controlled three-dimensional extracellular matrices for high-resolution imaging of cell behavior. Nat Protoc 7(11):2056–2066. https://doi.org/10.1038/nprot.2012.127
Gungordu HI, Bao M, van Helvert S, Jansen JA, Leeuwenburgh SCG, Walboomers XF (2019) Effect of mechanical loading and substrate elasticity on the osteogenic and adipogenic differentiation of mesenchymal stem cells. J Tissue Eng Regen Med 13(12):2279–2290. https://doi.org/10.1002/term.2956
Guo M, Pegoraro AF, Mao A, Zhou EH, Arany PR, Han Y, Burnette DT, Jensen MH, Kasza KE, Moore JR (2017a) Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proceedings of the National Academy of Sciences 114(41):E8618-E8627
Guo M, Pegoraro AF, Mao A, Zhou EH, Arany PR, Han Y, Burnette DT, Jensen MH, Kasza KE, Moore JR, Mackintosh FC, Fredberg JJ, Mooney DJ, Lippincott-Schwartz J, Weitz DA (2017b) Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc Natl Acad Sci USA 114(41):E8618–e8627. https://doi.org/10.1073/pnas.1705179114
Han P, Frith JE (2019) Five Piconewtons: The Difference between Osteogenic and Adipogenic Fate Choice in Human Mesenchymal Stem Cells. ACS Nano 13(10):11129–11143. https://doi.org/10.1021/acsnano.9b03914
Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ (2010) Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 9(6):518–526
Kang E-S, Kim D-S, Suhito IR, Lee W, Song I, Kim T-H (2018) Two-dimensional material-based bionano platforms to control mesenchymal stem cell differentiation. Biomaterials Res 22(1):10
Kim SM, Yi SW, Kim HJ, Park JS, Kim JH, Park KH (2019) Co-Delivery of RUNX2-Targeting miRNAs and shRNAs Using Nanoparticles Composed of Dexamethasone and PEI Induces Chondrogenesis of Human Mesenchymal Stem Cells. J Biomed Nanotechnol 15(1):113–126. https://doi.org/10.1166/jbn.2019.2671
Lima AF, May G, Díaz-Colunga J, Pedreiro S, Paiva A, Ferreira L, Enver T, Iborra FJ (2018) Osmotic modulation of chromatin impacts on efficiency and kinetics of cell fate modulation. 8:7210. https://doi.org/10.1038/s41598-018-25517-2. 1
Luo L, Foster NC, Man KL, Brunet M, Hoey DA, Cox SC, Kimber SJ, El Haj AJ (2022) Hydrostatic pressure promotes chondrogenic differentiation and microvesicle release from human embryonic and bone marrow stem cells. Biotechnol J 17(4):e2100401. https://doi.org/10.1002/biot.202100401
Magalhães J, Lebourg M, Deplaine H, Gómez Ribelles JL, Blanco FJ (2015) Effect of the physicochemical properties of pure or chitosan-coated poly(L-lactic acid)scaffolds on the chondrogenic differentiation of mesenchymal stem cells from osteoarthritic patients. Tissue Eng Part A 21(3–4):716–728. https://doi.org/10.1089/ten.TEA.2014.0133
Melnik S, Gabler J, Dreher SI, Hecht N, Hofmann N, Großner T, Richter W (2020) MiR-218 affects hypertrophic differentiation of human mesenchymal stromal cells during chondrogenesis via targeting RUNX2, MEF2C, and COL10A1. Stem Cell Res Ther 11(1):532. https://doi.org/10.1186/s13287-020-02026-6
Nalluri SM, Krishnan GR, Cheah C, Arzumand A, Yuan Y, Richardson CA, Yang S, Sarkar D (2015) Hydrophilic polyurethane matrix promotes chondrogenesis of mesenchymal stem cells. Materials science & engineering C, Materials for biological applications 54:182–195. https://doi.org/10.1016/j.msec.2015.05.043
Nancarrow-Lei R, Mafi P, Mafi R, Khan W (2017) A Systemic Review of Adult Mesenchymal Stem Cell Sources and their Multilineage Differentiation Potential Relevant to Musculoskeletal Tissue Repair and Regeneration. Curr Stem Cell Res Therapy 12(8):601–610. https://doi.org/10.2174/1574888x12666170608124303
Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T (2012) Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J BioChem 151(3):247–254. https://doi.org/10.1093/jb/mvs004
Olivares-Navarrete R, Lee EM, Smith K, Hyzy SL, Doroudi M, Williams JK, Gall K, Boyan BD, Schwartz Z (2017) Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 12(1):e0170312. https://doi.org/10.1371/journal.pone.0170312
Pötter N, Westbrock F, Grad S, Alini M, Stoddart MJ, Schmal H, Kubosch D, Salzmann G, Kubosch EJ (2021) Evaluation of the influence of platelet-rich plasma (PRP), platelet lysate (PL) and mechanical loading on chondrogenesis in vitro. Sci Rep 11(1):20188. https://doi.org/10.1038/s41598-021-99614-0
Pakfar A, Irani S, Hanaee-Ahvaz H (2017) Expressions of pathologic markers in PRP based chondrogenic differentiation of human adipose derived stem cells. Tissue Cell 49(1):122–130
Piroli ME, Jabbarzadeh E (2018) Matrix Stiffness Modulates Mesenchymal Stem Cell Sensitivity to Geometric Asymmetry Signals. Ann Biomed Eng 46(6):888–898. https://doi.org/10.1007/s10439-018-2008-8
Reinwald Y, El Haj AJ (2018) Hydrostatic pressure in combination with topographical cues affects the fate of bone marrow-derived human mesenchymal stem cells for bone tissue regeneration. J Biomedical Mater Res Part A 106(3):629–640
Ro H, Park J, Yang K, Kim J, Yim H-G, Jung G, Lee H, Cho S-W, Hwang NS (2015) Osteogenic priming of mesenchymal stem cells by chondrocyte-conditioned factors and mineralized matrix. Cell Tissue Res 362(1):115–126
Ruiz M, Maumus M, Fonteneau G, Pers Y-M, Ferreira R, Dagneaux L, Delfour C, Houard X, Berenbaum F, Rannou F (2019) TGFβi is involved in the chondrogenic differentiation of mesenchymal stem cells and is dysregulated in osteoarthritis. Osteoarthr Cartil 27(3):493–503
Shafaie S, Hutter V, Brown MB, Cook MT, Chau DY (2017) Influence of surface geometry on the culture of human cell lines: A comparative study using flat, round-bottom and v-shaped 96 well plates.PloS one12(10)
Srinivasan A, Chang SY, Zhang S, Toh WS, Toh YC (2018) Substrate stiffness modulates the multipotency of human neural crest derived ectomesenchymal stem cells via CD44 mediated PDGFR signaling. Biomaterials 167:153–167. https://doi.org/10.1016/j.biomaterials.2018.03.022
Sun M, Chi G, Li P, Lv S, Xu J, Xu Z, Xia Y, Tan Y, Xu J, Li L (2018) Effects of matrix stiffness on the morphology, adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells. Int J Med Sci 15(3):257
Tee SY, Fu J, Chen CS, Janmey PA (2011) Cell shape and substrate rigidity both regulate cell stiffness. Biophys J 100(5):L25–27. https://doi.org/10.1016/j.bpj.2010.12.3744
Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Stuart MAC, Boehm H, Li B, Vogel V (2012) Extracellular-matrix tethering regulates stem-cell fate. Nat Mater 11(7):642–649
Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ (2014) Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater 13(10):979–987. https://doi.org/10.1038/nmat4051
Wu Y, Yang Z, Law JB, He AY, Abbas AA, Denslin V, Kamarul T, Hui JH, Lee EH (2017) The Combined Effect of Substrate Stiffness and Surface Topography on Chondrogenic Differentiation of Mesenchymal Stem Cells. Tissue Eng Part A 23(1–2):43–54. https://doi.org/10.1089/ten.TEA.2016.0123
Xie J, Zhang D, Zhou C, Yuan Q, Ye L, Zhou X (2018a) Substrate elasticity regulates adipose-derived stromal cell differentiation towards osteogenesis and adipogenesis through β-catenin transduction. Acta Biomater 79:83–95. https://doi.org/10.1016/j.actbio.2018.08.018
Xie J, Zhang D, Zhou C, Yuan Q, Ye L, Zhou X (2018b) Substrate elasticity regulates adipose-derived stromal cell differentiation towards osteogenesis and adipogenesis through β-catenin transduction. Acta Biomater 79:83–95
Zhou C, Wang Q, Zhang D, Cai L, Du W, Xie J (2019a) Compliant substratum modulates vinculin expression in focal adhesion plaques in skeletal cells. Int J Oral Sci 11(2):1–9
Zhou C, Zhang D, Zou J, Li X, Zou S, Xie J (2019b) Substrate Compliance Directs the Osteogenic Lineages of Stem Cells from the Human Apical Papilla via the Processes of Mechanosensing and Mechanotransduction. 11:26448–26459. https://doi.org/10.1021/acsami.9b07147. 29
Zhou C, Zhang D, Zou J, Li X, Zou S, Xie J (2019c) Substrate Compliance Directs the Osteogenic Lineages of Stem Cells from the Human Apical Papilla via the Processes of Mechanosensing and Mechanotransduction. ACS Appl Mater Interfaces 11(29):26448–26459
Funding
The project was supported by the National Natural Science Foundation of China (31971240).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Juan Li and Jingyi Qiu designed the experiments. Jingyi Qiu and Lingyun Wan performed the experiments. Yimei Zhou and Jingyi Qiu analyzed the experimental results. Yimei Zhou and Lingyun Wan wrote the manuscript with help from all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors declare that they have no conflicts of interest to this work, financial or otherwise.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Qiu, J., Wan, L. et al. The effect of matrix stiffness on the chondrogenic differentiation of mesenchymal stem cells. J Mol Histol 53, 805–816 (2022). https://doi.org/10.1007/s10735-022-10094-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-022-10094-6