Abstract
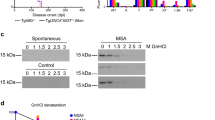
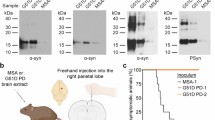
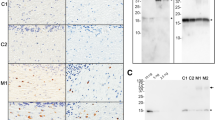
In multiple system atrophy (MSA), the protein α-synuclein misfolds into a prion conformation that self-templates and causes progressive neurodegeneration. While many point mutations in the α-synuclein gene, SNCA, have been identified as the cause of heritable Parkinson’s disease (PD), none have been identified as causing MSA. To examine whether MSA prions can transmit disease to mice expressing wild-type (WT) human α-synuclein, we inoculated transgenic (Tg) mice denoted TgM20+/− with brain homogenates prepared from six different deceased MSA patients. All six samples transmitted CNS disease to the mice, with an average incubation period of ~ 280 days. Interestingly, TgM20+/− female mice developed disease > 60 days earlier than their male counterparts. Brains from terminal mice contained phosphorylated α-synuclein throughout the hindbrain, consistent with the distribution of α-synuclein inclusions in MSA patients. In addition, using our α-syn–YFP cell lines, we detected α-synuclein prions in brain homogenates prepared from terminal mice that retained MSA strain properties. To our knowledge, the studies described here are the first to show that MSA prions transmit neurological disease to mice expressing WT SNCA and that the rate of transmission is sex dependent. By comparison, TgM20+/− mice inoculated with WT preformed fibrils (PFFs) developed severe neurological disease in ~ 210 days and exhibited robust α-synuclein neuropathology in both limbic regions and the hindbrain. Brain homogenates from these animals exhibited biological activities that are distinct from those found in MSA-inoculated mice when tested in the α-syn–YFP cell lines. Differences between brains from MSA-inoculated and WT PFF-inoculated mice potentially argue that α-synuclein prions from MSA patients are distinct from the PFF inocula and that PFFs do not replicate MSA strain biology.







Similar content being viewed by others
References
Bernis ME, Babila JT, Breid S, Wüsten KA, Wüllner U, Tamgüney G (2015) Prion-like propagation of human brain-derived alpha-synuclein in transgenic mice expressing human wild-type alpha-synuclein. Acta Neuropathol Commun 3:75
Carlson GA, Kingsbury DT, Goodman PA, Coleman S, Marshall ST, DeArmond S et al (1986) Linkage of prion protein and scrapie incubation time genes. Cell 46:503–511
Conway KA, Harper JD, Lansbury PT (1998) Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med 4:1318–1320
Coon EA, Nelson RM, Sletten DM, Suarez MD, Ahlskog JE, Benarroch EE et al (2019) Sex and gender influence symptom manifestation and survival in multiple system atrophy. Auton Neurosci 219:49–52
Dhillon J-S, Trejo-Lopez JA, Riffe C, Levites Y, Sacino AN, Borchelt DR et al (2019) Comparative analyses of the in vivo induction and transmission of α-synuclein pathology in transgenic mice by MSA brain lysate and recombinant α-synuclein fibrils. Acta Neuropathol Commun 7:80
Emmer KL, Waxman EA, Covy JP, Giasson BI (2011) E46K human alpha-synuclein transgenic mice develop Lewy-like and tau pathology associated with age-dependent, detrimental motor impairment. J Biol Chem 286:35104–35118
Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM (2002) Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34:521–533
Giasson BI, Uryu K, Trojanowski JQ, Lee VM (1999) Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 274:7619–7622
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Guerrero-Ferreira R, Taylor NM, Mona D, Ringler P, Lauer ME, Riek R et al (2018) Cryo-EM structure of alpha-synuclein fibrils. eLife 7:e36402
Hass EW, Sorrentino ZA, Lloyd GM, McFarland NR, Prokop S, Giasson BI (2021) Robust α-synuclein pathology in select brainstem neuronal populations is a potential instigator of multiple system atrophy. Acta Neuropathol Commun 9:80
Jellinger KA, Lantos PL (2010) Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol 119:657–667
Korth C, Kaneko K, Groth D, Heye N, Telling G, Mastrianni J et al (2003) Abbreviated incubation times for human prions in mice expressing a chimeric mouse—human prion protein transgene. Proc Natl Acad Sci USA 100:4784–4789
Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM et al (2010) Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet 19:1633–1650
Lau A, So RWL, Lau HHC, Sang JC, Ruiz-Riquelme A, Fleck SC et al (2020) α-Synuclein strains target distinct brain regions and cell types. Nat Neurosci 23:21–31
Li B, Ge P, Murray KA, Sheth P, Zhang M, Nair G et al (2018) Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat Commun 9:3609
Li Y, Zhao C, Luo F, Liu Z, Gui X, Luo Z et al (2018) Amyloid fibril structure of α-synuclein determined by cryo-electron microscopy. Cell Res: https://doi.org/10.1038/s41422-018-0075-x
Lloyd GM, Sorrentino ZA, Quintin S, Gorion KM, Bell BM, Paterno G et al (2022) Unique seeding profiles and prion-like propagation of synucleinopathies are highly dependent on the host in human α-synuclein transgenic mice. Acta Neuropathol 143:663–685
Long H, Zheng W, Liu Y, Sun Y, Zhao K, Liu Z et al (2021) Wild-type α-synuclein inherits the structure and exacerbated neuropathology of E46K mutant fibril strain by cross-seeding. Proc Natl Acad Sci USA 118:e2012435118
Luk KC, Covell DJ, Kehm VM, Zhang B, Song IY, Byrne MD et al (2016) Molecular and biological compatibility with host alpha-synuclein influences fibril pathogenicity. Cell Rep 16:3373–3387
Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D et al (1999) Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J Biol Chem 274:9843–9846
Ni X, McGlinchey RP, Jiang J, Lee JC (2019) Structural insights into α-synuclein fibril polymorphism: effects of Parkinson’s disease-related C-terminal truncations. J Mol Biol 431:3913–3919
Nishie M, Mori F, Yoshimoto M, Takahashi H, Wakabayashi K (2004) A quantitative investigation of neuronal cytoplasmic and intranuclear inclusions in the pontine and inferior olivary nuclei in multiple system atrophy. Neuropathol Appl Neurobiol 30:546–554
Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94:79–100
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A et al (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047
Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB et al (2015) Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci USA 112:E5308–E5317
Rosborough K, Patel N, Kalia LV (2017) α-Synuclein and parkinsonism: updates and future perspectives. Curr Neurol Neurosci Rep 17:31
Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M et al (1998) Eight prion strains have PrPSc molecules with different conformations. Nat Med 4:1157–1165
Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M (1998) Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 251:205–208
Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M (1997) α-Synuclein in Lewy bodies. Nature 388:839–840
Telling GC, Scott M, Hsiao KK, Foster D, Yang S-L, Torchia M et al (1994) Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc Natl Acad Sci USA 91:9936–9940
Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen FE et al (1995) Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83:79–90
Wakabayashi K, Miki Y, Tanji K, Mori F (2022) Neuropathology of multiple system atrophy, a glioneuronal degenerative disease. Cerebellum. https://doi.org/10.1007/s12311-022-01407-2
Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM et al (2013) Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci USA 110:19555–19560
Woerman AL, Kazmi SA, Patel S, Aoyagi A, Oehler A, Widjaja K et al (2018) Familial Parkinson’s point mutation abolishes multiple system atrophy prion replication. Proc Natl Acad Sci USA 115:409–414
Woerman AL, Kazmi SA, Patel S, Freyman Y, Oehler A, Aoyagi A et al (2018) MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol 135:49–63
Woerman AL, Oehler A, Kazmi SA, Lee J, Halliday GM, Middleton LT et al (2019) Multiple system atrophy prions retain strain specificity after serial propagation in two different Tg(SNCA*A53T) mouse lines. Acta Neuropathol 137:437–454
Woerman AL, Patel S, Kazmi SA, Oehler A, Lee J, Mordes DA et al (2020) Kinetics of α-synuclein prions preceding neuropathological inclusions in multiple system atrophy. PLOS Pathog 16:e1008222
Woerman AL, Stöhr J, Aoyagi A, Rampersaud R, Krejciova Z, Watts JC et al (2015) Propagation of prions causing synucleinopathies in cultured cells. Proc Natl Acad Sci USA 112:E4949–E4958
Acknowledgements
We thank Dr. Benoit Giasson (University of Florida) for kindly providing the TgM20 mice, Jeffrey Lau for his assistance with experiments, and the Hunters Point animal facility staff for breeding and caring for the animals used in this study. We also thank Dr. Deborah Mash (Miami Brain Bank) for providing control tissue. This work was supported by grants from the National Institutes of Health (NIH) (P01AG002132) as well as support by the Sergey Brin Foundation and the Sherman Fairchild Foundation (S.B.P.). It was also supported by grants from the NIH (R01NS121294) and the CurePSP Foundation (668-2020-06), and by the University of Massachusetts Amherst (A.L.W.). The Parkinson’s UK Brain Bank at Imperial College London is funded by Parkinson’s UK, a charity registered in England and Wales (948776) and in Scotland (SC037554). The Massachusetts Alzheimer’s Disease Research Center is supported by the NIH (AG005134).
Author information
Authors and Affiliations
Contributions
SAMH, SHO, SBP, and ALW designed the research; SAMH, JL, AO, FKO, and ALW performed the research; DAM contributed new reagents; SAMH, JL, SBP, and ALW analyzed the data; and SBP and ALW wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
S.B.P is the founder of Prio-Pharma, which has not contributed financial or any other support to these studies. A.L.W. is a member of Acta Neuropathologica’s Editorial Board. They were not involved in the assessment or decision-making process for this manuscript.
Ethical approval
Animals were maintained in an AAALAC-accredited facility in compliance with the 8th Guide for the Care and Use of Laboratory Animals. All procedures used in this study were approved by the University of California San Francisco Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Holec, S.A.M., Lee, J., Oehler, A. et al. Multiple system atrophy prions transmit neurological disease to mice expressing wild-type human α-synuclein. Acta Neuropathol 144, 677–690 (2022). https://doi.org/10.1007/s00401-022-02476-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02476-7