Abstract
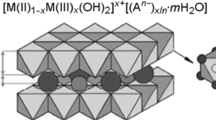
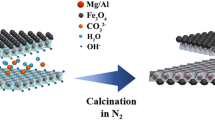
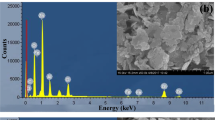
Layered double hydroxides (LDHs) have been extensively studied as adsorbents and the outer surface of their layers plays an important role in the global process of adsorption. Arsenic is one of the most important water contaminants, and in addition to its geological occurrence, it is discarded in the nature, for instance, from pesticides and mining. In this work, an LDH magnesium-aluminum intercalated by carbonate ions was prepared on the magnetite core by the co-precipitation method and the equilibrium and thermodynamic aspects were evaluated in the arsenic removal from water. The magnetic core facilitated the adsorbent recovery by the simple application of an external magnetic field, which may be a strategy to enhance its reusability. The synthesized adsorbent was characterized by Fourier transform infrared spectroscopy (FTIR), powder X-ray diffraction (PXRD) and point of zero charge (pHPZC) techniques. The crystalline profile of the LDH phase was kept in the composites formed by a magnetic core. As expected, the characterization analyses evidenced the intercalation of the carbonate ion, and the pHPZC was 8.02, a crucial parameter with regard to the balance of chemical forms of arsenic-containing ions and their dependence on the pH of the reaction medium. The adsorption reaction was endergonic, exothermic and presented negative standard entropy. The Dubinin−Radushkevich isotherm was applied and the adsorption energy (E) was 1.36 kJ mol–1, indicating that the process is ruled by physical forces. Sips and Radke−Prausnitz isotherms were able to fit the experimental data. The maximum adsorption capacity (qm) was 23 mg g–1, which is lower than the expected if the LDH phase had acted in the removal of arsenic ions using its outer surface plus its capacity of ion exchange, and this step was highly influenced by the pH of the arsenic-containing solution. In addition, as the temperature rose, the equilibrium constant decreased whereas the qm increased, indicating that the temperature has favored the arsenic species diffusion inside the pores of the LDH aggregates. The Mag-LDH presented potential to be further studied as an alternative adsorbent for arsenic removal from water.




Similar content being viewed by others
REFERENCES
Dinwiddie, E. and Liu, X.-M., Examining the geologic link of arsenic contamination in groundwater in Orange County, North Carolina, Front. Earth Sci., 2018, vol. 6. https://doi.org/10.3389/feart.2018.00111
Abdul, K.S.M., Jayasinghe, S.S., Chandana, E.P.S., Jayasumana, C., and de Silva, P.M.C.S., Arsenic and human health effects: A review, Environ. Toxicol. Pharmacol., 2015, vol. 40, pp. 828–846. https://doi.org/10.1016/j.etap.2015.09.016
Yogarajah, N. and Tsai, S.S.H., Detection of trace arsenic in drinking water: Challenges and opportunities for microfluidics, Environ. Sci. Water Res. Technol., 2015, vol. 1, pp. 426–447. https://doi.org/10.1039/C5EW00099H
Masuda, H., Arsenic cycling in the Earth’s crust and hydrosphere: Interaction between naturally occurring arsenic and human activities, Prog. Earth Planet. Sci., 2018, vol. 5, pp. 68–79. https://doi.org/10.1186/s40645-018-0224-3
Hao, L., Liu, M., Wang, N., and Li, G., A critical review on arsenic removal from water using iron-based adsorbents, RSC Adv., 2018, vol. 8, pp. 39545–39560. https://doi.org/10.1039/c8ra08512a
Johnston, A.-L., Lester, E., Williams, O., and Gomes, R.L., Understanding layered double hydroxide properties as sorbent materials for removing organic pollutants from environmental waters, J. Environ. Chem. Eng., 2021, vol. 9, 105197. https://doi.org/10.1016/j.jece.2021.105197
Mishra, G., Dash, B., and Pandey, S., Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials, Appl. Clay Sci., 2018, vol. 153, pp. 172–186. https://doi.org/10.1016/j.clay.2017.12.021
Sasai, R., Sato, H., Sugata, M., Fujimura, T., Ishihara, S., Deguchi, K., Ohki, S., Tansho, M., Shimizu, T., Oita, N., Numoto, M., Fujii, Y., Kawaguchi, S., Matsuoka, Y., Hagura, K., Abe, T., and Moriyoshi, C., Why do carbonate anions have extremely high stability in the interlayer space of layered double hydroxides? Case study of layered double hydroxide consisting of Mg and Al (Mg/Al = 2), Inorg. Chem., 2019, vol. 58, pp. 10928–10935. https://doi.org/10.1021/acs.inorgchem.9b01365
Leung, K., Nielsen, I.M.B., and Criscenti, L.J., Elucidating the bimodal acid-base behavior of the water-silica interface from first principles, J. Am. Chem. Soc., 2009, vol. 131, pp. 18358–18356. https://doi.org/10.1021/ja906190t
Lowe, B.M., Skylaris, C.-K., and Green, N.G., Acid-base dissociation mechanisms and energetics at the silica-water interface: An activationless process, J. Colloid Interface Sci., 2015, vol. 451, pp. 231–244. https://doi.org/10.1016/j.jcis.2015.01.094
El-Reesh, G.Y., Farghali, A.A., Taha, M., and Mahmoud, R.K., Novel synthesis of Ni/Fe layered double hydroxides using urea and glycerol and their enhanced adsorption behavior for Cr(VI) removal, Sci. Rep., 2020, vol. 10, pp. 587–607. https://doi.org/10.1038/s41598-020-57519-4
Hou, L., Liang, Q., and Wang, F., Mechanisms that control the adsorption-desorption behavior of phosphate on magnetite nanoparticles: The role of particle size and surface chemistry characteristics, RSC Adv., 2020, vol. 10, pp. 2378–2388. https://doi.org/10.1039/c9ra08517c
Rashid, H., Mansoor, M.A., Haider, B., Nasir, R., Abd Hamid, S.B., and Abdulrahman, A., Synthesis and characterization of magnetite nano particles with high selectivity using in-situ precipitation method, Sep. Sci. Technol., 2019, vol. 55, pp. 1207–1215. https://doi.org/10.1080/01496395.2019.1585876
Wijitwongwan (Ploy), R., Intasa-ard (Grace), S., and Ogawa, M., Preparation of layered double hydroxides toward precisely designed hierarchical organization, Chem. Eng., 2019, vol. 3, pp. 68–90. https://doi.org/10.3390/chemengineering3030068
Suponik, T., Winiarski, A., and Szade, J., Processes of removing zinc from water using zero-valent iron, Water, Air, Soil Pollut., 2015, vol. 226, pp. 360–371. https://doi.org/10.1007/s11270-015-2617-x
Okazaki, T., Wang, W., Kuramitz, H., Hata, N., and Taguchi, S., Molybdenum blue spectrophotometry for trace arsenic in ground water using a soluble membrane filter and calcium carbonate column, Anal. Sci., 2013, vol. 29, pp. 67–72. https://doi.org/10.2116/analsci.29.67
Mahjoubi, F.Z., Khalidi, A., Abdennouri, M., and Barka, N., Zn–Al layered double hydroxides intercalated with carbonate, nitrate, chloride and sulphate ions: Synthesis, characterization and dye removal properties, J. Taibah Univ. Sci., 2017, vol. 11, pp. 90–100. https://doi.org/10.1016/j.jtusci.2015.10.007
Amelia, T., Saputro, R.E., Hidayat, N., Taufiq, A., Diantoro, M., Sunaryono, S., Mufti, N., and Hidayat, A., Synthesis and structural characterization of iron chromite nanoparticles: A preliminary study, J. Phys.: Conf. Ser., 2020, vol. 1595, pp. 12027–12034. https://doi.org/10.1088/1742-6596/1595/1/012027
Bhojaraj Arulraj, J., Kolinjavadi, M.R., and Rajamathi, M., Solvent-mediated and echanochemical methods for anion exchange of carbonate from layered double hydroxides using ammonium salts, ACS Omega, 2019, vol. 4, pp. 20072–20079. https://doi.org/10.1021/acsomega.9b03261
Türk, T., Alp, I., and Deveci, H., Adsorption of As(V) from water using Mg–Fe-based hydrotalcite (FeHT), J. Hazard. Mater., 2009, vol. 171, pp. 665–670. https://doi.org/10.1016/j.jhazmat.2009.06.052
Ghosal, P.S. and Gupta, A.K., An insight into thermodynamics of adsorptive removal of fluoride by calcined Ca–Al–(NO3) layered double hydroxide, RSC Adv., 2015, vol. 5, 105889. https://doi.org/10.1039/C5RA20538G
Kovačević, D., Njegić, B.N., Hasenay, D., Nemet, I., Rončević, S., Dékány, I., and Petridise, D., Adsorption of arsenic on MgAl layered double hydroxide, Croat. Chem. Acta, 2013, vol. 86, pp. 273–279. https://doi.org/10.5562/cca2283
Ayawei, N., Ebelegi, A.N., and Wankasi, D., Modelling and interpretation of adsorption isotherms, J. Chem., 2017, vol. 2017, 3039817. https://doi.org/10.1155/2017/3039817
Monárrez-Cordero, B.E., Amézaga-Madrid, P., Leyva-Porras, C.C., Pizá-Ruiza, P., and Miki-Yoshida, M., Study of the adsorption of arsenic(III and V) by magnetite nanoparticles synthetized via AACVD, Mater. Res., 2016, vol. 19, pp. 103–112. https://doi.org/10.1590/1980-5373-mr-2015-0667
Park, J.-Y. and Kim, J.-H., Characterization of adsorbed arsenate on amorphous and nano crystalline MgFe-layered double hydroxides, J. Nanopart. Res., 2011, vol. 13, pp. 887–894. https://doi.org/10.1007/s11051-010-9936-z
ACKNOWLEDGMENTS
We are grateful to the Universidade Estadual de Londrina for the characterization analysis.
Funding
The work was supported by Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), and Fundacao Araucaria do Parana (Parana Araucaria Foundation).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Luciane Effting, Alesandro Bail The “Plus” of the Outer Surface of a Magnetic Layered Double Hydroxide for Arsenic Removal from Water: Synthesis and Adsorption Aspects. J. Water Chem. Technol. 44, 250–258 (2022). https://doi.org/10.3103/S1063455X22040063
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X22040063