Abstract
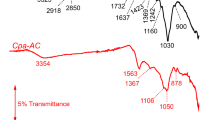
N/O-rich porous carbon adsorbent was synthesized from coffee powder residue collected from the nearby cafeteria using pyrolysis at 200°C. The adsorbents were used for Ni adsorption using synthetic Ni-incorporated water. The structural, morphological, surface chemistry information of the sample was extracted using various characterization techniques. The two peaks at 23.3° and 41.1° in the X-ray diffraction pattern confirm the graphitic nature of carbon, whereas the third peak at 12.1° reveals the co-existence of certain graphitic oxide in the sample. The IR spectrum indicates the presence of N-containing (NH) functional groups on the virgin sample. A shift of band positions corresponding to NH and OH vibrations indicates adsorbed Ni(II) interaction with the sample. Experiment optimization of input variables by one factor at a time model was used to optimize three experimental parameters: solution pH, initial Ni(II) concentration (IC), and adsorbent dosage for higher Ni(II) removal and optimum time using Response surface methodology. The adsorption studies of the adsorbent toward Ni(II) removal from aqueous solution were performed using response surface methodology by optimizing adsorption parameters. According to the analysis of variance, a lack of fit of 1.38 is estimated, which validates the model very well. Analysis of variance techniques was used for experimental validation. The maximum removal efficiency of 97.6% is achieved at pH 7 for initial concentration 5.5 mg/L and adsorbent weight 0.2 g in 50 mL solution for 52 min. pHzpc of the sample was determined as 7 in agreement with the experiment confirming thereby the optimum pH for Ni(II) adsorption. Adsorption data were well fitted to Langmuir adsorption isotherm indicating a homogeneous monolayer adsorption of Ni(II) on the sample. The hydroxyl and amine functional groups on the sorbent form Ni complex via coordinate bonding with Ni(II). For Ni(II) content <9 mg/L, the adsorbent dosage and contact time can be optimized for a removal efficiency of 90–100%.




Similar content being viewed by others
Change history
17 October 2022
An Erratum to this paper has been published: https://doi.org/10.3103/S1063455X22050137
REFERENCES
Fu, F. and Wang, Q., Removal of heavy metal ions from wastewaters: A review, J. Environ. Manage., 2011, vol. 92, pp. 407–418.
Facts and Figures—Water Pollution is on the Rise Globally, UNESCO WWAP, 2017. http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/facts-and-figures/all-facts-wwdr3/fact-15-water-pollution/. Accessed February 5, 2021.
Water and Agenda 2030, UNESCO WWAP, 2019. http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/water-and-agenda-2030/. Accessed February 5, 2021.
Hussain, J., Husain, I., Arif, M., and Gupta, N., Studies on heavy metal contamination in Godavari river basin, Appl. Water Sci., 2017, vol. 7, pp. 4539–4548.
Rizvi, A., Parveen, S., Khan, S., and Naseem, I., Nickel toxicology with reference to male molecular reproductive, Physiol. Reprod. Biol., 2020, vol. 20, no. 1, pp. 3–8.
Ali, I., Alharbi, O.M.L., ALOthman, Z.A., Al-Mohaimeed, A.M., and Alwarthan, A., Modeling of fenuron pesticide adsorption on CNTs for mechanistic insight and removal in water, Environ. Res., 2019, vol. 170, pp. 389–397.
Nezamzadeh-Ejhieh, A. and Kabiri-Samani, M., Effective removal of Ni(II) from aqueous solutions by modification of nano particles of clinoptilolite with dimethylglyoxime, J. Hazard. Mater., 2013, vol. 260, pp. 339–349.
Adolph, M.A., Xavier, Y.M., Kriveshini, P., and Rui, K., Phosphine functionalized multiwalled carbon nanotubes: A new adsorbent for the removal of nickel from aqueous solution, J. Environ. Sci., 2012, vol. 24, no. 6, pp. 1133–1141.
Babel, S. and Kurniawan, T.A., Low-cost adsorbents for heavy metals uptake from contaminated water: A review, J. Hazard. Mater., 2003, vol. 97, pp. 219–243.
Asuquo, E., Martin, A., Nzerem, P., Siperstein, F., and Fan, X.L., Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterization studies, J. Environ. Chem. Eng., 2017, vol. 5, no. 1, pp. 679–698.
Niazi, N.K., Murtaza, B., Bibi, I., Shahid, M., White, J.C., Nawaz, M.F., Bashir, S., Shakoor, M.B., Choppala, G., Murtaza, G., and Wang, H., Removal and Recovery of Metals by Biosorbents and Biochars Derived From Biowastes, in Environmental Materials and Waste: Resource Recovery and Pollution Prevention, Prasad, M.N.V. and Shih, K., Eds., New York: Academic, 2016, pp. 149–177.
Wong, S., Ngadi, N., Inuwa, I.M., and Hassan, O., Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review, J. Cleaner Prod., 2018, vol. 175, pp. 361–375.
Sen, S., Nandi, S., and Dutta, S., Application of RSM and ANN for optimization and modelling of biosorption of chromium(VI) using cyanobacterial biomass, Appl. Water Sci., 2018, vol. 8, 148.
Zhu, L., Gao, Q., Tan, Y., Tian, W., Xu, J., Yang, K., and Yang, C., Nitrogen and oxygen co-doped microporous carbons derived from the leaves of Euonymus japonicas as high-performance supercapacitor electrode material, Microporous Mesoporous Mater., 2015, vol. 210, pp. 1–9.
Maharana, H.S., Rai, P.K., and Basu, A., Surface-mechanical and electrical properties of pulse electrodeposited Cu-graphene oxide composite coating for electrical contacts, J. Mater. Sci., 2017, vol. 52, pp. 1089–1105.
Liu, R. and Lian, B., Non-competitive and competitive adsorption of Cd2+, Ni2+, and Cu2+ by biogenic vaterite, Sci. Total Environ., 2019, vol. 659, pp. 122–130.
Feng, Z., Li, Z., Zhang, X., Shi, Y., and Zhou, N., Nitrogen-doped carbon quantum dots as fluorescent probes for sensitive and selective detection of nitrite, Molecules., 2017, vol. 22, no. 12, 2061.
Grube, M., Muter, O., Strikauska, S., Gavare, M., and Limane, B., Application of FT-IR for control of the medium composition during biodegradation of nitroaromatic compounds, J. Ind. Microbiol. Biotechnol., 2008, vol. 35, pp. 1545–1549.
Wang, S., Jin, X., Zhao, H., and Wu, F., Phosphate biosorption characteristics of a submerged macrophyte Hydrilla verticillate, Aquat. Bot., 2008, vol. 89, no. 1, pp. 23–26.
Mezenner, N.Y. and Bensmaili, A., Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide eggshell waste, Chem. Eng. J., 2009, vol. 147, pp. 87–96.
Trakoolsa, O. and Yoochatchavl, W., Adsorption efficiency of copper and nickel by activated carbon from coffee ground, Environ. Asia, 2020, vol. 13, special issue, pp. 46–53.
Shah, I., Adnan, R., Ngah, W.S.W., and Mohamed, N., Iron impregnated activated carbon as an efficient adsorbent for the removal of Methylene Blue: Regeneration and kinetics studies, PLoS One, 2015, vol. 10, no. 4, e0122603.
Hasar, H., Adsorption of Ni(II) from aqueous solution onto activated carbon prepared from almond husk, J. Hazard. Mater., 2003, vol. 97, pp. 49–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Padma Seragadam, Lakshmi, P.J., Naveen, P. et al. N/O-Rich Porous Carbon Adsorbent from Coffee-Residue toward Ni(II) Removal from Surface Water. J. Water Chem. Technol. 44, 241–249 (2022). https://doi.org/10.3103/S1063455X22040117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X22040117