Abstract
Background
Patients with severe psoriasis are prone to deterioration of renal function. Whether biologics with potent anti-inflammatory action can prevent deterioration of renal function in psoriatic patients was unclear.
Objective
To investigate the effects of different biologics on renal function in patients with severe psoriasis.
Methods
By using the Chang Gung Research Database in Taiwan during 2006–2018, we analyzed the changes in renal function of psoriatic patients from 2 years before biologic treatments to baseline (start of biologic treatment) to after 2 years’ treatment with different classes of biologics (anti-TNF, anti-IL-12/23, and anti-IL-17 agents). The renal function was evaluated by estimated glomerular filtration rate (eGFR) and the staging of chronic kidney disease (CKD). We further analyzed the risk factors of progression on the staging of CKD during biologics treatment.
Results
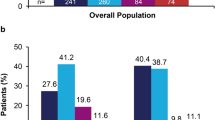
We included 601 patients with severe psoriasis receiving continuous use of biologics for ≥ 2 years. We detected no significant differences between pre-biologic treatment with conventional systemic treatment and post-biologic treatment in the levels of eGFR and progression of CKD staging among psoriatic patients receiving different classes of biologics. Most patients (97.8%) remained at stable CKD stage, while progression of CKD stage over time occurred in 13 patients (2.2%), with seven treated with anti-TNF biologics and six treated with anti-IL-12/23 biologics. Of note, all 52 patients receiving anti-IL-17 biologics had stable CKD. Progression of CKD during biologics use was associated with lower baseline levels of eGFR, higher baseline CKD stage, older age, diabetes, and dyslipidemia. Further multiple logistic regression analysis showed diabetes as an independent factor for the deterioration of renal function during biologic treatment.
Conclusions
Biologic treatments failed to improve but did not worsen renal function of psoriatic patients during a 2-year follow-up period. Diabetes is an important risk factor for the deterioration of renal function.


Similar content being viewed by others
References
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Identification, Management of P, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–85.
Kemeriz F, Tuğrul B, Tuncer S. C-reactive protein to albumin ratio: Is a new parameter for the disease severity in patients with psoriasis vulgaris? Dermatol Sin. 2020;38(4):199–204.
Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163(3):586–92.
Fang TP, Lin YC, Chi CC. Association of psoriasis with asthma: a systematic review and meta-analysis of observational studies. Dermatol Sin. 2020;38(1):22–7.
Demirci O, Ates B, Sagaltici E, Ocak Z, Altunay I. Association of the attachment styles with depression, anxiety, and quality of life in patients with psoriasis. Dermatol Sin. 2020;38(2):81–7.
Spah F. Inflammation in atherosclerosis and psoriasis: common pathogenic mechanisms and the potential for an integrated treatment approach. Br J Dermatol. 2008;159(Suppl 2):10–7.
Ng CY, Huang YH, Tzeng IS, Liu SH, Chang YC. Changes in metabolic parameters in psoriasis patients treated with interleukin-12/23 blockade (ustekinumab). Dermatol Sin. 2020;38(3):166–71.
Puig L. Cardiometabolic comorbidities in psoriasis and psoriatic arthritis. Int J Mol Sci. 2017;19(1):58.
Chen W, Chen W, Wang H, Dong X, Liu Q, Mao H, et al. Prevalence and risk factors associated with chronic kidney disease in an adult population from southern China. Nephrol Dial Transplant. 2009;24(4):1205–12.
Cecchi R, Seghieri G, Gironi A, Tuci F, Giomi A. Relation between urinary albumin excretion and skin involvement in patients with psoriasis. Dermatology. 1992;185(2):93–5.
Szepietowski JC, Bielicka E, Wasik F, Kopec W, Szepietowski T. Microalbuminuria as a subclinical marker of renal impairment in subjects with psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2000;14(6):513–4.
Dervisoglu E, Akturk AS, Yildiz K, Kiran R, Yilmaz A. The spectrum of renal abnormalities in patients with psoriasis. Int Urol Nephrol. 2012;44(2):509–14.
Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population-based study. Br J Dermatol. 2011;165(5):1037–43.
Wan J, Wang S, Haynes K, Denburg MR, Shin DB, Gelfand JM. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347: f5961.
Chi CC, Wang J, Chen YF, Wang SH, Chen FL, Tung TH. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: a nationwide population-based cohort study. J Dermatol Sci. 2015;78(3):232–8.
Yang SF, Chen TH, Tsai SH, Chen PE, Chi CC, Tung TH. Risk of chronic kidney disease and end-stage renal disease in patients with psoriasis: a systematic review and meta-analysis of cohort studies. Dermatol Sin. 2021;39(1):19–26.
Krebs CF, Turner JE, Riedel JH, Panzer U. Tissue-specific therapy in immune-mediated kidney diseases: new ARGuments for targeting the IL-23/IL-17 axis. J Clin Invest. 2021;131(12):e150588.
Andrade-Oliveira V, Foresto-Neto O, Watanabe IKM, Zatz R, Camara NOS. Inflammation in renal diseases: new and old players. Front Pharmacol. 2019;10:1192.
Mehaffey E, Majid DSA. Tumor necrosis factor-alpha, kidney function, and hypertension. Am J Physiol Renal Physiol. 2017;313(4):F1005–8.
Li H, Tsokos MG, Bhargava R, Adamopoulos IE, Menn-Josephy H, Stillman IE, et al. IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation. J Clin Invest. 2021;131(12):e142428.
Schmidt T, Luebbe J, Kilian C, Riedel JH, Hiekmann S, Asada N, et al. IL-17 receptor C signaling controls CD4(+) TH17 immune responses and tissue injury in immune-mediated kidney diseases. J Am Soc Nephrol. 2021;32(12):3081–98.
Maghfour J, Elliott E, Gill F, Stumpf B, Murina A. Effect of biologic drugs on renal function in psoriasis patients with chronic kidney disease. J Am Acad Dermatol. 2020;82(5):1249–51.
Tsai MS, Lin MH, Lee CP, Yang YH, Chen WC, Chang GH, et al. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed J. 2017;40(5):263–9.
Shao SC, Chan YY, Kao Yang YH, Lin SJ, Hung MJ, Chien RN, et al. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28(5):593–600.
Wang SH, Chi CC, Hu S. Cost-efficacy of biologic therapies for moderate to severe psoriasis from the perspective of the Taiwanese healthcare system. Int J Dermatol. 2014;53(9):1151–6.
Tsai TF, Lee CH, Huang YH, Chi CC, Chang YT, Wong TW, et al. Taiwanese Dermatological Association consensus statement on management of psoriasis. Dermatol Sin. 2017;35(2):66–77.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54.
Uhlig K, Macleod A, Craig J, Lau J, Levey AS, Levin A, et al. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int. 2006;70(12):2058–65.
Gisondi P, Girolomoni G. Glomerular filtration rate in patients with psoriasis treated with etanercept. J Int Med Res. 2016;44(1 suppl):106–8.
Gisondi P, Pezzolo E, Girolomoni G. Glomerular filtration rate in patients with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2019;33(6):e244–6.
Hueber AJ, Tunc A, Schett G, Manger B. Anti-tumour necrosis factor alpha therapy in patients with impaired renal function. Ann Rheum Dis. 2007;66(7):981–2.
Nimmannitya K, Tateishi C, Mizukami Y, Hamamoto K, Yamada S, Goto H, et al. Successful treatment with ustekinumab of psoriasis vulgaris in a patient undergoing hemodialysis. J Dermatol. 2016;43(1):92–4.
Mikhaylov D, Hashim PW, Nektalova T, Goldenberg G. Systemic psoriasis therapies and comorbid disease in patients with psoriasis: a review of potential risks and benefits. J Clin Aesthet Dermatol. 2019;12(6):46–54.
Ikonomidis I, Papadavid E, Makavos G, Andreadou I, Varoudi M, Gravanis K, et al. Lowering interleukin-12 activity improves myocardial and vascular function compared with tumor necrosis factor-a antagonism or cyclosporine in psoriasis. Circ Cardiovasc Imaging. 2017;10(9):e006283.
Makavos G, Ikonomidis I, Andreadou I, Varoudi M, Kapniari I, Loukeri E, et al. Effects of interleukin 17a inhibition on myocardial deformation and vascular function in psoriasis. Can J Cardiol. 2020;36(1):100–11.
Cortvrindt C, Speeckaert R, Moerman A, Delanghe JR, Speeckaert MM. The role of interleukin-17A in the pathogenesis of kidney diseases. Pathology. 2017;49(3):247–58.
Lavoz C, Matus YS, Orejudo M, Carpio JD, Droguett A, Egido J, et al. Interleukin-17A blockade reduces albuminuria and kidney injury in an accelerated model of diabetic nephropathy. Kidney Int. 2019;95(6):1418–32.
Amoruso GF, Nistico SP, Iannone L, Russo E, Rago G, Patruno C, et al. Ixekizumab may improve renal function in psoriasis. Healthcare (Basel). 2021;9(5):543.
Ribero S, Conti V, Gambardella A, Der AB, Dapavo P, Argenziano G. Brodalumab treatment in psoriasis patients with severe renal failure. Ital J Dermatol Venerol. 2021;156(3):406–8.
Mastorino L, Roccuzzo G, Dapavo P, Siliquini N, Avallone G, Rubatto M, et al. Patients with psoriasis resistant to multiple biological therapies: characteristics and definition of a difficult-to-treat population. Br J Dermatol. 2022;187(2):263–5.
Acknowledgements
The authors acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (CLRPG3D0048) at Chang Gung Memorial Hospital, Linkou for statistical consultation and data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by research grants from the Chang Gung Memorial Hospital, Linkou, Taiwan (CORPG3J0061). The funder played no role in study design, data collection, data analysis, manuscript preparation, and publication decisions.
Data availability statement
The data used for this study are available from the corresponding author on reasonable request.
Conflicts of interest
None.
Ethics approval
This study was approved by the Institutional Review Board of Chang Gung Medical Foundation (IRB. NO. 201802089B0C502).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability:
Not applicable.
Author contributions
CBC, TYH, CCH, SHC, and CCC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: CBC, CCH, SHC, and CCC. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: CBC, TYH, CCH, and CCC. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: CBC, TYH, SHC, and CCC. Obtained funding: SHC, and CCC. Study supervision: CCC.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, CB., Huang, YT., Hsiao, CC. et al. Real-World Effects of Biologics on Renal Function in Psoriatic Patients: A Retrospective Study. BioDrugs 36, 657–666 (2022). https://doi.org/10.1007/s40259-022-00547-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00547-5