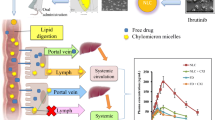
Abstract
Andrographolide, the primary bioactive constituent of Andrographis paniculata, is a promising natural substance with numerous pharmacotherapy uses. Low water solubility, short half-life, and low permeability necessitate the development of a delivery system that enhances its entrapment efficiency, bioavailability, lymphatic targeting, and by-pass hepatic effect. The andrographolide-loaded solid lipid nanoparticles were fabricated by melt-emulsification and ultrasonication and optimized with Design-Expert software. In the optimal formulation, Glycerol monostearate as the solid lipid and Poloxamer 407 and Span 60 as surfactants were used. Optimum AND-SLN was observed to have a mean particle size, polydispersity index, zeta potential, and entrapment efficiency of 193.84 nm, 0.211, − 22.8 mV, and 83.70% respectively. An optimized formulation was characterized by examining surface morphology, X-ray diffraction, and differential scanning calorimetry. In vitro studies have shown sustained drug release from AND-SLN for up to 24 h. The stability studies showed that there was no significant change in the mean particle size and entrapment efficiency after storage at 4 ± 2 °C and 25 ± 2 °C/60 ± 5% RH. In in vivo pharmacokinetics studies, AND-SLN was found to have enhanced bioavailability and specificity in the spleen and thymus compared to plasma, providing evidence that the formulations could enhance target specificity and bioavailability in comparison to pure drugs. The H&E staining of the liver, spleen, and thymus treated with the AND-SLN revealed no signs of damage histopathologically. Thus, AND-SLN possess a high potential for improved efficacy and are an efficient vehicle for delivering drugs to the lymphatic system.
Graphical abstract








Similar content being viewed by others
Availability of data and materials
NA.
Abbreviations
- AND:
-
Andrographolide
- AND-SLN:
-
Andrographolide-loaded solid lipid nanoparticles
- AND-Susp:
-
Suspension of andrographolide
- BBB:
-
Box–Behnken design
- CPCSEA:
-
The Committee for the Purpose of Control and Supervision of Experiments on Animals
- DLS:
-
Dynamic light scattering
- DSC:
-
Differential scanning calorimetry
- EE:
-
Entrapment efficiency
- FT-IR:
-
Fourier transform infrared spectroscopy
- GMS:
-
Glycerol monostearate
- H&E:
-
Hematoxylin and eosin
- HPLC:
-
High-performance liquid chromatography
- ICH:
-
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- PDI:
-
Polydispersity index
- PS:
-
Particle size
- RH:
-
Relative humidity
- rpm:
-
Revolutions per minute
- SD:
-
Standard deviation
- SEM:
-
Scanning electron microscopy
- SLNs:
-
Solid lipid nanoparticles
- TEM:
-
Transmission electron microscopy
- UV:
-
Ultraviolet
- XRD:
-
X-ray diffraction
- ZP:
-
Zeta potential
References
Zhongyu X, Jiangmeng R, Qiufang J, et al. Andrographolide-loaded silk fibroin nanoparticles. RSC Adv. 2018;8(16):34726–32.
Jiang Y, Wang F, Xu H, et al. Development of andrographolide loaded PLGA microspheres: optimization, characterization and in vitro–in vivo correlation. Int J Pharm. 2014;475(1):475–84.
Jayakumar T, Hsieh CY, Lee JJ, et al. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid-based Complement. Altern Med. 2013;1–17.
Sajeeb BK, Kumar U, Halder S, et al. Identification and quantification of andrographolide from Andrographis paniculata (Burm. f.) Wall. ex Nees by RP-HPLC method and standardization of its market preparations. Dhaka Univ J Pharma Sci. 2015;14(1):71–8.
Casamonti M, Risaliti L, Vanti G, et al. Andrographolide loaded in micro-and nano-formulations: improved bioavailability, target-tissue distribution, and efficacy of the “king of bitters.” Engrg. 2019;5(1):69–75.
Parveen R, Ahmad FJ, Iqbal Z, et al. Solid lipid nanoparticles of anticancer drug andrographolide: formulation, in vitro and in vivo studies. Drug Dev Ind Pharm. 2014;40(9):1206–12.
Graverini G, Piazzini V, Landucci E, et al. Solid lipid nanoparticles for delivery of andrographolide across the blood-brain barrier: in vitro and in vivo evaluation. Colloids Surf B: Biointerfaces. 2018;161:302–13.
Ali M, Afzal M, Kaushik U, Bhattacharya SM, Ahmad FJ, Dinda AK. Perceptive solutions to anti-filarial chemotherapy of lymphatic filariasis from the plethora of nanomedical sciences. J Drug Targeting. 2014;22(1):1–3.
Shrivastava S, Kaur CD. Perception of lymphatic system and their disorders. Br J Pharm Med Res. 2018;3(4):1119–31.
Khan AA, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomed. 2013;8:2733.
Shrivastava S, Gidwani B, Gupta A, Kaur CD. Ethnopharmacological approaches to treat lymphatic filariasis. Int J Pharm Ana Res. 2016;5(3):445–70.
Velmurugan R, Selvamuthukumar S. Development and optimization of ifosfamide nanostructured lipid carriers for oral delivery using response surface methodology. Appl Nanosci. 2016;6(2):159–73.
Makwana V, Jain R, Patel K, et al. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495(1):439–46.
Shrivastava S, Gidwani B, Kaur CD. Development of mebendazole loaded nanostructured lipid carriers for lymphatic targeting: optimization, characterization, in-vitro and in-vivo evaluation. Part Sci Technol. 2021;39(3):380–90.
Shrivastava S, Gupta A, Kaur CD. The epitome of novel techniques and targeting approaches in drug delivery for treating lymphatic filariasis. Curr Drug Targets. 2020;21(12):1250–63.
Gidwani B, Vyas A. Preparation, characterization, and optimization of altretamine-loaded solid lipid nanoparticles using Box-Behnken design and response surface methodology. Artif Cells Nanomed Biotechnol. 2016;44(2):571–80.
Rapalli VK, Kaul V, Waghule T, et al. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur J Pharm Sci. 2020;152: 105438.
El Assasy AE, Younes NF, Makhlouf AI. Enhanced oral absorption of amisulpride via a nanostructured lipid carrier-based capsule: development, optimization applying the desirability function approach and in vivo pharmacokinetic study. AAPS PharmSciTech. 2019;20(2):1–4.
Alcantara KP, Zulfakar MH, Castillo AL. Development, characterization and pharmacokinetics of mupirocin-loaded nanostructured lipid carriers (NLCs) for intravascular administration. Int J Pharm. 2019;571: 118705.
Makeen HA, Mohan S, Al-Kasim MA, et al. Gefitinib loaded nanostructured lipid carriers: characterization, evaluation and anti-human colon cancer activity in vitro. Drug Deliv. 2020;27(1):622–31.
Mishra A, Vuddanda PR, Singh S. Intestinal lymphatic delivery of praziquantel by solid lipid nanoparticles: formulation design, in vitro and in vivo studies. J Nanotechnol 2014;1–13.
Duong VA, Nguyen TT, Maeng HJ, et al. Preparation of ondansetron hydrochloride-loaded nanostructured lipid carriers using solvent injection method for enhancement of pharmacokinetic properties. Pharm Res. 2019;36(10):1–12.
Teng Z, Yu, M Ding Y, et al. Preparation and characterization of nimodipine-loaded nanostructured lipid systems for enhanced solubility and bioavailability. Int J Nanomed. 2019;14:119.
Ijaz M, Akhtar N. Fatty acids based α-Tocopherol loaded nanostructured lipid carrier gel: In vitro and in vivo evaluation for moisturizing and anti-aging effects. J Cosmet Dermatol. 2020;19(11):3067–76.
Soni K, Rizwanullah M, Kohli K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: in vitro, ex vivo and in vivo assessments. Artif Cells Nanomed Biotechnol. 2018;46(1):15–31.
Qumber M, Alruwaili NK, Bukhari SN, Alharbi KS, Imam SS, Afzal M, Alsuwayt B, Mujtaba A, Ali A. BBD-based development of itraconazole loaded nanostructured lipid carrier for topical delivery: in vitro evaluation and antimicrobial assessment. J Pharm Innov. 2021 Mar;16(1):85–98.
Gidwani B, Vyas A. Formulation, characterization and evaluation of cyclodextrin-complexed bendamustine-encapsulated PLGA nanospheres for sustained delivery in cancer treatment. Pharm Dev Technol. 2016;21(2):161–71.
Negi LM, Talegaonkar S, Jaggi M, et al. Surface engineered nanostructured lipid carriers for targeting MDR tumor: part II. In vivo biodistribution, pharmacodynamic and hematological toxicity studies. Colloids Surf B: Biointerfaces. 2014;123:610–15.
Ganesan P, Narayanasamy D. Lipid nanoparticles: different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm. 2017;6:37–56.
Elmowafy M, Ibrahim HM, Ahmed MA, et al. Atorvastatin-loaded nanostructured lipid carriers (NLCs): strategy to overcome oral delivery drawbacks. Drug Deliv. 2017;24(1):932–41.
Alex MA, Chacko AJ, Jose S, et al. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci. 2011;42(1):11–8.
Kumar S, Narayan R, Ahammed V, et al. Development of ritonavir solid lipid nanoparticles by Box Behnken design for intestinal lymphatic targeting. J Drug Deliv Sci Technol. 2018;44:181–9.
Kaur CD, Nahar M, Jain NK. Lymphatic targeting of zidovudine using surface-engineered liposomes. J Drug Target. 2008;16(10):798–805.
Ahammed V, Narayan R, Paul J, et al. Development and in vivo evaluation of functionalized ritonavir proliposomes for lymphatic targeting. Life Sci. 2017;183:11–20.
Zeng K, Li J, Zhang Z, et al. Lipid-coated ZnO nanoparticles as lymphatic-targeted drug carriers: study on cell-specific toxicity in vitro and lymphatic targeting in vivo. J Mater Chem B. 2015;3(26):5249–60.
Chen J, Jiang H, Wu Y, et al. A novel glycyrrhetinic acid-modified oxaliplatin liposome for liver-targeting and in vitro/vivo evaluation. Drug Des Devel Ther. 2015;9:2265.
Gurumukhi VG, Bari SB. Quality by design (QbD)–based fabrication of atazanavir-loaded nanostructured lipid carriers for lymph targeting: bioavailability enhancement using chylomicron flow block model and toxicity studies. Drug Deliv Transl Res. 2021;1–23.
Chiang CS, Huang BJ, Chen YJ, et al. Fucoidan-based nanoparticles with inherently therapeutic efficacy for cancer treatment. Pharmaceutics. 2021;13(12):1986.
Shrivastava S, Daharwal SJ. Box-Behnken design avenue for development and validation of high-performance thin-layer chromatography method for estimation of rutin in Hemidesmus indicus. Biomed Chromatogr. 2022;36(1): e5236.
Acknowledgements
The authors are grateful for the facilities provided by the Management and Principal of SRIP, Kumhari. We also recognize everyone who helped us conduct this research directly and indirectly.
Funding
The Chhattisgarh Council of Science & Technology in the form of a grant from the CCOST/MRP/1106/2015 and the CCOST/MRP/1120/2015 project, CGCOST, Raipur, Chhattisgarh, India, provided financial help for conducting these research projects.
Author information
Authors and Affiliations
Contributions
Both authors contributed to framing the idea, studying the conception, and designing the manuscript. Conceptualization: (Saurabh Shrivastava), (Chanchal Deep Kaur); Methodology: (Saurabh Shrivastava); Software: (Saurabh Shrivastava); Formal analysis and investigation: (Saurabh Shrivastava); Writing—original draft preparation: (Saurabh Shrivastava), (Chanchal Deep Kaur); Writing—review and editing: (Saurabh Shrivastava), (Chanchal Deep Kaur); Funding acquisition: (Chanchal Deep Kaur); Resources: (Saurabh Shrivastava), (Chanchal Deep Kaur); Supervision: (Chanchal Deep Kaur).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Experimental protocols were approved by the Institutional Animal Ethical Committee (SRIP/IAEC/2019–20/191/07), and studies were conducted under CPCSEA guidelines. Human subjects were not involved in this study, so there was no need for informed consent from the participants. All institutional and national guidelines for the care and use of laboratory animals were followed.
Consent for publication
NA.
Competing interests
The authors declare no competing interests.
Disclosure statement
The work originated in our lab. There is no conflict of interest among writers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Nanocarriers can improve the therapeutic profile of AND.
• The AND-SLN was prepared by melt-emulsification ultrasonication and optimized by Design-Expert software.
• An optimized formulation was characterized by DSC, XRD, and TEM.
• AND-SLN showed excellent in vitro and in vivo characteristics.
• AND-SLN is efficacious carriers of drugs targeting the lymphatic system with a high potential for improved efficacy.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shrivastava, S., Kaur, C.D. Development of andrographolide-loaded solid lipid nanoparticles for lymphatic targeting: Formulation, optimization, characterization, in vitro, and in vivo evaluation. Drug Deliv. and Transl. Res. 13, 658–674 (2023). https://doi.org/10.1007/s13346-022-01230-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01230-6