Abstract
Objective
Central sleep apnea (CSA) is associated with increased morbidity and mortality in patients with heart failure (HF). We aimed to explore the effectiveness of phrenic nerve stimulation (PNS) on CSA in patients with HF.
Methods
This was a prospective and non-randomized study. The stimulation lead was inserted into the right brachiocephalic vein and attached to a proprietary neurostimulator. Monitoring was conducted during the implantation process, and all individuals underwent two-night polysomnography.
Results
A total of nine subjects with HF and CSA were enrolled in our center. There was a significant decrease in the apnea–hypopnea index (41 ± 18 vs 29 ± 25, p = 0.02) and an increase in mean arterial oxygen saturation (SaO2) (93% ± 1% vs 95% ± 2%, p = 0.03) after PNS treatment. We did not observe any significant differences of oxygen desaturation index (ODI) and SaO2 < 90% (T90) following PNS. Unilateral phrenic nerve stimulation might also categorically improve the severity of sleep apnea.
Conclusion
In our non-randomized study, PNS may serve as a therapeutic approach for CSA in patients with HF.
Similar content being viewed by others
Introduction
Central sleep apnea (CSA) is common in subjects with heart failure (HF), affecting almost half of subjects with systolic HF and 18 to 30% of subjects with diastolic HF [1,2,3,4,5]. CSA is mainly caused by increased respiratory response to variations in PaCO2. This oscillation is caused by heightened respiratory instability. Hyperventilation, circulatory delay, and enhanced cerebrovascular reactivity are three elements that determine respiratory instability in patients with HF [6]. CSA can lead to hypoxia, consequences of increases in arrhythmias and sympathetic drive [7, 8]. In subjects with HF, it has been shown to be an important risk factor for mortality [9].
Phrenic nerve stimulation (PNS) is a new method of treating CSA in HF patients by preserving the physiological breathing pattern during central apnea episodes [10, 11]. Using an implantable device therapy is easier than mask-based positive pressure therapies for patients with HF, then improving therapeutic adherence. The PNS treatment has been supported to be an effective treatment in a previous randomized controlled trial involving 151 patients [12]. Although this new device has been used in a few medical centers around the world, it seems to be a safe and effective approach for treating CSA. Ponikowski et al. [13]. conducted a prospective, non-randomized trial to determine the feasibility of PNS for the treatment of CSA in patients with HF. Thirty-one patients from six centers were selected; 16 of them were able to undergo two nights of polysomnography (PSG). They found PNS could result in significant improvement in the AHI and central apnea index(CAI), and PNS can significantly decrease the incidence of CSA and bring back a more natural breathing pattern in patients with HF. Zhang X et al. [14] showed a significant reduction in AHI and CAI at 6-month follow-up.
Previous studies [12,13,14] have demonstrated that this treatment is safe, that it can significantly reduce episodes of CSA, and that it provides improvements in crucial polysomnographic indicators. However, studies on the effectiveness of applied PNS in HF patients with CSA are still scarce; therefore, our study provides strong evidence for the effectiveness of PNS.
Methods
Participants and data collection
The Remedē System Pivotal Trial is a short-term, prospective, single-center, open-label trial involving patients with CSA. Patients who had a diagnosis of sleep apnea and/or previous polysomnographic (PSG) tests supporting periodic breathing with CSA within the preceding 6 months were eligible for this short-term trial. The participants were then subjected to two more full nights of PSG by study design. Subjects were enrolled if they had an apnea–hypopnea index (AHI) ≥ 15. Patients who had supplementary oxygen, phrenic nerve palsy, severe COPD, unstable angina within 3 months of the study, or poor phrenic nerve capture during neurostimulation were excluded in this study.
Procedure description
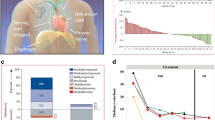
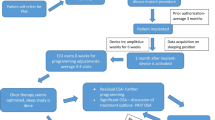
The axillary or subclavian veins were used to gain venous access. To activate the nearby phrenic nerve, stimulation leads (Cardima catheter, USA) were placed in the right brachiocephalic vein (Fig. 1). Low-energy nerve stimulation was delivered by an external pulse generator device (Respicardia, Inc.). Capture was determined during the lead implantation operation by external palpation of diaphragmatic contraction on the stimulation side. The level of phrenic nerve stimulation is adjusted as required throughout the evening session, aiming to eliminate centrally mediated apnea episodes that do not disturb the subject.
Scoring of polysomnographic studies
Two qualified sleep technicians evaluated the two-night PSG. Subject identities, study night ordering and stimulating application were blinded from the technicians. An episode of apnea was characterized as a deficiency of inspiratory airflow over 10 s. Obstructive apnea (OA) was defined as a lack of airflow in the presence of rib and abdominal excursions. Central apnea (CA) was defined as a lack of airflow in the absence of rib and abdominal excursions, as well as a lack of airflow. Hypopnea was defined as a drop in airflow that lasted 10 s or longer accompanied by a drop of at least 4% in arterial oxyhemoglobin saturation.
Statistical analysis
Descriptive statistics are expressed as standard deviation or numbers and percentages. Paired t tests (for data with a normal distribution) and Wilcoxon tests (for data with an abnormal distribution) were performed before and after treatment. Results were considered statistically significant at p < 0.05. Data were analyzed using SPSS version 25.0 (New York, USA).
Results
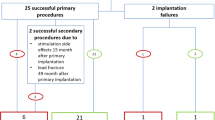
The characteristics of these nine subjects were summarized in Table 1. Subjects in this study were all male (aged 74.4 ± 8.4 years with a body mass index (BMI) of 28.7 ± 3.5 kg/m2). They received standard treatment for HF, and their mean left ventricular ejection fraction (LVEF) was 43 ± 14%. The stimulation lead was positioned in the right brachiocephalic vein for all patients. Three individuals had previously installed cardiac devices. Three patients had a device for cardiac resynchronization treatment (CRT). In the context of PNS, devices were examined for probable over- or under-sensing. To evaluate potential disturbances, the implanted device was programmed with the greatest sensitivity level.
The PNS led to significant improvements in the severity of CSA, including decreased AHI (p = 0.02) (Fig. 2 and Table 2). After PNS, mean SaO2 was increased significantly (p = 0.03) in these individuals (Table 2). Using established categories of disease severity based on AHI, categorical reductions were also noted in the severity of sleep apnea following unilateral PNS treatment (Table 3). During the two-night trial follow-up, no significant adverse events occurred in our research.
Discussion
CSA, typically associated with symptomatic HF, is widely observed in clinical practice and associated with a poor outcome. Currently, PAP is the standard treatment for CSA. Clinical trials using PAP for treating CSA have yielded contradictory outcomes [15, 16]. The CANPAP trial was a randomized, outcome study that assessed the efficacy of CPAP treatment for CSA in HF subjects; this study revealed no benefits of CPAP [17]. A post hoc examination of the trial’s data suggested that mortality could be reduced if CPAP therapy is associated with an early and considerable reduction in AHI. The mean AHI in the adaptive servo-ventilation (ASV) group at 12 months was 6.6 e/h [18]. The incidence of the primary endpoint was not substantially different between the ASV and control groups. In the ASV group, overall mortality and cardiovascular mortality were considerably greater than in the control group [19]. When compared with PAP, the benefit of PNS includes a natural breathing pattern by a diaphragmatic stimulation. As a result, physiological effects of diaphragmatic stimulation do not have similar negative hemodynamic effects on cerebral hemodynamics as PAP breathing (e.g., increased intrathoracic pressure affecting right and left ventricular preload and afterload) [20,21,22]. Prospective self-controlled studies such as PNS comparison can be recommended to the same patient, with a pause after PAP treatment [23]. The recent approval of the PNS system in Europe and USA provides new hope for patients with CSA. Fudim et al. used pooled individual data from the pilot (n = 57) and pivotal (n = 151) studies of the Remedē System in patients with predominant moderate to severe CSA. At 6 months, PNS reduced AHI by a median of − 22.6 e/h (25th and 75th percentiles; − 38.6 and − 8.4, respectively); PNS decreases CSA severity and sleep quality considerably. Significant and long-term reductions in key predictors of CSA severity, such as AHI, CAI, and 4% ODI, established the feasibility and therapeutic efficacy of PNS for CSA [24,25,26]. The degree of AHI and the reduction in AHI are related, for example, to improved outcomes in patients with obstructive or CSA. It remains to be determined whether reductions in crucial sleep parameters, symptoms, and heart function by the Remedē System can have a positive effect on cardiovascular results [18, 27]. Implant success and procedural complication rates were improved from the pilot study to the pivotal phase. Increased operator experience, improved leads, and updated implantation techniques may contribute to the rate of implant success [28].
Our study showed that PNS can be utilized to treat CSA in individuals with HF, leading to a substantial decrease in AHI. Those with the most severe sleep apnea, as defined by an AHI > 30 e/h, have the highest mortality rates [29]. In our study, the proportion of subjects with severe sleep apnea decreased from 66 to 44% when PNS was administered. During the therapeutic night, five patients (55%) had an AHI < 15 e/h. Previous research divided 151 suitable patients into treatment (n = 73) or control (n = 78) groups. Six months later, those in the treatment group had an AHI reduction from baseline that was higher than or equal to 55%, whereas those in the control group did not achieve this reduction. Significant improvement in reducing the severity of CSA, improvements in arousal indices as well as in rapid eye movement sleep, PGA scores, and ESS were observed with PNS. Consistent improvements in oxygenation and quality of life support the clinical relevance of this therapy, making PNS a potential treatment for CSA [25]. The results of the trial showed that only two patients were unable to adapt to the treatment. The therapy was well tolerated. The first implantation success rate was very high. Despite lead dislodgement, it was comparable to other implantable devices using the transvenous lead technique. A total of 138 (91%) of 151 patients experienced no serious-related side events at 12 months [25]. Our study did not detect an increased mortality in HF patients after PNS; however, past studies have shown a signal of increased mortality in CSA patients treated with PNS [19, 30]. In Dariusz’s research, only five major adverse events occurred during the 12 months of follow-up. There were no deaths as a result of serious adverse events linked to the device or procedure. None of these incidents were fatal [31]. The safety of any new medical device must be evaluated over time.
Our study had limitations. This is a single-center and non-randomized trial. We only evaluated a two-night therapy with a limited sample size, and all patients were men (a high prevalence of CSA in male patients with HF). The design of the study did not allow us to fully evaluate the potential complications of this therapy, such as its potential to interfere with pre-existing implanted cardiac devices. In addition, a few individuals were excluded due to issues with the lead placement. Notably, many patients with HF who have CSA may also have obstructive apnea; future randomized, controlled trials are needed to obtain stronger evidence.
Conclusion
In our non-randomized study, the use of unilateral transvenous PNS may reduce the severity of CSA, providing a new approach for the treatment of CSA in patients with HF.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V (2007) Sleep disordered breathing in patients with symptomatic heart failure. A contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 9:251–257
Javaheri S (2006) Sleep disorders in systolic heart failure: a prospective study of 100 male patients The final report. Int J Cardiol 106:21–28
Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD (1999) Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 160:1101–1106
Macdonald M, Fang J, Pittman SD, White DP, Malhotra A (2008) The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 04:38–42
Herrscher TE, Akre H, Øverland B, Sandvik L, Westheim AS (2011) High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Cardiac Fail 17:420–425
Costanzo Maria R, Khayat R, Ponikowski P, Augostini R, Stellbrink C, Mianulli M, Abraham William T (2015) Mechanisms and clinical consequences of untreated central sleep apnea in heart failure. J Am Coll Cardiol 65:72–84
Lanfranchi PA, Somers VK, Braghiroli A, Corra U, Eleuteri E, Giannuzzi P (2003) Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation 107:727–732
Garcia-Touchard A, Somers VK, Olson LJ, Caples SM (2008) Central sleep apnea: implications for congestive heart failure. Chest 133:1495–1504
Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD (2000) Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation 102:61–66
Cao M, Guilleminault C (2012) Sleep-disordered breathing, heart failure, and phrenic nerve stimulation. Chest 142:821–823
Costanzo MR, Ponikowski P, Coats A, Javaheri S, Augostini R, Goldberg LR, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C, McKane S, Abraham AT, S Remede System Pivotal Trial (2018) Phrenic nerve stimulation to treat patients with central sleep apnoea and heart failure. Eur J Heart Fail 20:1746–1754
Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C (2016) Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 388:974–982
Ponikowski P, Javaheri S, Michalkiewicz D, Bart BA, Czarnecka D, Jastrzebski M, Kusiak A, Augostini R, Jagielski D, Witkowski T (2012) Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 33:889–894
Zhang X, Ding N, Ni B, Yang B, Wang H, Zhang SJ (2017) Safety and feasibility of chronic transvenous phrenic nerve stimulation for treatment of central sleep apnea in heart failure patients. Clin Respir J 11:176–184
Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, Neal B (2017) Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA 318:156–166
Cowie MR, Wegscheider K, Teschler H (2016) Adaptive servo-ventilation for central sleep apnea in heart failure. N Engl J Med 374:690–691
Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS (2005) Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 353:2025–2033
Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Ryan C, Tomlinson G, Bradley TD (2007) Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 115:3173–3180
Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho M-P, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F (2015) Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 373:1095–1105
Le Pimpec-Barthes F, Gonzalez-Bermejo J, Hubsch J-P, Duguet A, Morélot-Panzini C, Riquet M, Similowski T (2011) Intrathoracic phrenic pacing: a 10-year experience in France. J Thorac Cardiovasc Surg 142:378–383
Combes N, Jaffuel D, Cayla G, Granier M, Borel JC, Corne P, Jonquet O, Jaber S, Davy JM, Pépin JL (2014) Pressure-dependent hemodynamic effect of continuous positive airway pressure in severe chronic heart failure: a case series. Int J Cardiol 171:e104–e105
Liston R, Deegan P, McCreery C, Costello R, Maurer B, McNicholas W (1995) Haemodynamic effects of nasal continuous positive airway pressure in severe congestive heart failure. Eur Respir J 8:430–435
Borel J-C, Gakwaya S, Masse J-F, Melo-Silva CA, Sériès F (2012) Impact of CPAP interface and mandibular advancement device on upper airway mechanical properties assessed with phrenic nerve stimulation in sleep apnea patients. Respir Physiol Neurobiol 183:170–176
Abraham WT, Jagielski D, Oldenburg O, Augostini R, Krueger S, Kolodziej A, Gutleben KJ, Khayat R, Merliss A, Harsch MR, Holcomb RG, Javaheri S, Ponikowski P (2015) Phrenic nerve stimulation for the treatment of central sleep apnea, JACC. Heart failure 3:360–369
Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C, Abraham WT (2016) Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet (London, England) 388:974–982
Fudim M, Spector AR, Costanzo MR, Pokorney SD, Mentz RJ, Jagielski D, Augostini R, Abraham WT, Ponikowski PP, McKane SW, Piccini JP (2019) Phrenic nerve stimulation for the treatment of central sleep apnea: a pooled cohort analysis. J Clin Sleep Med 15:1747–1755
Jilek C, Krenn M, Sebah D, Obermeier R, Braune A, Kehl V, Schroll S, Montalvan S, Riegger GA, Pfeifer M, Arzt M (2011) Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail 13:68–75
Linde C, Abraham WT, Gold MR, St. John Sutton M, Ghio S, Daubert C, R.S Group (2008) Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 52:1834–1843
Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM (2008) Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31:1071–1078
Costanzo MR, Ponikowski P, Coats A, Javaheri S, Augostini R, Goldberg LR, Holcomb R, Kao A, Khayat RN, Oldenburg O (2018) Phrenic nerve stimulation to treat patients with central sleep apnoea and heart failure. Eur J Heart Fail 20:1746–1754
Jagielski D, Ponikowski P, Augostini R, Kolodziej A, Khayat R, Abraham WT (2016) Transvenous stimulation of the phrenic nerve for the treatment of central sleep apnoea: 12 months’ experience with the remedē® System. Eur J Heart Fail 18:1386–1393
Acknowledgements
Youmeng Wang is a mentee of World Sleep Society’s International Sleep Research Training Program (ISRTP) 2021.
Funding
Open Access funding enabled and organized by Projekt DEAL. Youmeng Wang was supported by the China Scholarship Council (CSC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was conducted according to the Declaration of Helsinki. The study was approved by the ethics committee of Charité University Hospital, Germany.
Consent to participate
Written informed consent was provided from each participant.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thomas Penzel—although the co-author is one of the two editors-in-chief of the journal, there was no involvement with the peer review process for this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Schoebel, J., Han, J. et al. Phrenic nerve stimulation for the treatment of central sleep apnea in patients with heart failure. Sleep Breath 27, 1027–1032 (2023). https://doi.org/10.1007/s11325-022-02699-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-022-02699-8