Abstract
Objective
To standardize a method for 1H MRS intramuscular absolute quantification of carnosine in the thigh, using a surface coil and water as internal reference.
Materials and methods
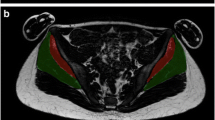
Carnosine spectra were acquired in phantoms (5, 10, and 15 mM) as well as in the right gastrocnemius medialis (GM) and right vastus lateralis (VLM) muscles of young team sports athletes, using volume (VC) and surface (SC) coils on a 3 T scanner, with the same receiver gain. Water spectra were used as internal reference for the absolute quantification of carnosine.
Results
Phantom’s experiments showed a maximum error of 7%, highlighting the validity of the measurements in the study setup. The carnosine concentrations (mmol/kg ww, mean ± SD) measured in the GM were 6.8 ± 2.2 with the VC (CcarVC) and 10.2 ± 3.0 with the SC (CcarSC) (P = 0.013; n = 9). Therefore, a correction was applied to these measurements (CcarVC = 0.6582*CcarSC), to make coils performance comparable (6.8 ± 2.2 for VC and 6.7 ± 2.0 for SC, P = 0.97). After that, only the SC was used to quantify carnosine in the VLM, where a concentration of 5.4 ± 1.5 (n = 30) was found, with significant differences between men (6.2 ± 1.3; n = 15) and women (4.6 ± 1.2; n = 15). The error in quantitation was 5.3–5.5% with both coils.
Conclusion
The method using the SC and water as internal reference can be used to quantify carnosine in voluminous muscles and regions of the body in humans, where the VC is not suitable, such as the VLM.




Similar content being viewed by others
References
Boldyrev A, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013;93:1803–45.
Fresta C, Fidilio A, Lazzarino G, Musso N, Grasso M, Merlo S, et al. Modulation of pro-oxidant and pro-inflammatory activities of M1 macrophages by the natural dipeptide carnosine. Int J Mol Sci. 2020;21:776.
Menon K, Marquina C, Liew D, Mousa A, de Courten B. Histidine-containing dipeptides reduce central obesity and improve glycaemic outcomes: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2020;21: e12975.
de Courten B, Kurdiova T, de Courten MP, Belan V, Everaert I, Vician M, et al. Muscle carnosine is associated with cardiometabolic risk factors in humans. PLoS ONE. 2015;10: e0138707.
Wu J, Egusa A, Nishimura T. Carnosine synthase deficiency in mice affects protein metabolism in skeletal muscle. Biochem Biophys Res Commun. 2022;612:22–9.
Harris R, Dunnett M, Greenhaff P. Carnosine and taurine contents in individual fibres of human vastus lateralis muscle. J Sport Sci. 1998;16:639–43.
Tallon M, Harris R, Maffulli N, Tarnopolsky M. Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology. 2007;8:129–37.
Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73:195–262.
Kenney W, Wilmore J, Costill D. Structure and function of exercising muscle. Physiol Sport Exerc. 6th ed. Champaign, USA: Human Kinetics; 2015. p. 27–50.
Baguet A, Bourgois J, Vanhee L, Achten E, Derave W. Important role of muscle carnosine in rowing performance. J Appl Physiol. 2010;109:1096–101.
Baguet A, Everaert I, Hespel P, Petrovic M, Achten E, Derave W. A new method for non-invasive estimation of human muscle fiber type composition. PLoS ONE. 2011;6: e21956.
Derave W, Ozdemir M, Harris R, Pottier A, Reyngoudt H, Koppo K, et al. beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J Appl Physiol. 2007;103:1736–43.
Kukurová IJ, Valkovič L, Ukropec J, de Courten B, Chmelík M, Ukropcová B, et al. Improved spectral resolution and high reliability of in vivo (1) H MRS at 7 T allow the characterization of the effect of acute exercise on carnosine in skeletal muscle. NMR Biomed. 2016;29:24–32.
Ozdemir M, Reyngoudt H, De Deene Y, Sazak H, Fieremans E, Delputte S, et al. Absolute quantification of carnosine in human calf muscle by proton magnetic resonance spectroscopy. Phys Med Biol. 2007;52:6781–94.
Londoño F, Calderón JC, Gallo J. Association between thigh muscle development and the metabolic syndrome in adults. Ann Nutr Metab. 2012;61:41–6.
Hayes C, Axel L. Noise performance of surface coils for magnetic resonance imaging at 1.5 T. Med Phys. 1985;12:604–7.
da Eira Silva V, de SallesPainelli V, Shinjo SK, Ribeiro Pereira W, Cilli EM, Sale C, et al. Magnetic resonance spectroscopy as a non-invasive method to quantify muscle carnosine in humans: a comprehensive validity assessment. Sci Rep. 2020;10:4908.
de Graaf R. Basic principles. Vivo NMR Spectrosc Princ Tech. 2nd Ed. Chichester, UK: John Wiley & Sons; 2007. p. 1–42.
Helms G. The principles of quantification applied to in vivo proton MR spectroscopy. Eur J Radiol. 2008;67:218–29.
Hoult D, Richards R. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J Magn Reson. 1976;24:71–85.
Krššák M, Lindeboom L, Schrauwen-Hinderling V, Szczepaniak LS, Derave W, Lundbom J, et al. Proton magnetic resonance spectroscopy in skeletal muscle: experts’ consensus recommendations. NMR Biomed. 2021;34: e4266.
Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–86.
Barkhuijsen H, de Beer R, van Ormondt D. Improved algorithm for noniterative time-domain model fitting to exponentially damped magnetic resonance signals. J Magn Reson. 1987;73:553–7.
Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43.
Krššák M, Mlynárik V, Meyerspeer M, Moser E, Roden M. 1H NMR relaxation times of skeletal muscle metabolites at 3 T. MAGMA. 2004;16:155–9.
Kreis R, Ernst T, Ross B. Absolute quantitation of water and metabolites in the human brain. II. Metabolite concentrations. J Magn Reson Ser B. 1993;102:9–19.
Ward S, Lieber R. Density and hydration of fresh and fixed human skeletal muscle. J Biomech. 2005;38:2317–20.
Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Bland J, Altman D. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–7.
Mannion A, Jakeman P, Willan P. Skeletal muscle buffer value, fibre type distribution and high intensity exercise performance in man. Exp Physiol. 1995;80:89–101.
Parkhouse W, McKenzie D, Hochachka P, Ovalle W. Buffering capacity of deproteinized human vastus lateralis muscle. J Appl Physiol. 1985;58:14–7.
Aldini G, Orioli M, Rossoni G, Savi F, Braidotti P, Vistoli G, et al. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J Cell Mol Med. 2011;15:1339–54.
Gualano B, Everaert I, Stegen S, Artioli G, Taes Y, Roschel H, et al. Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids. 2012;43:21–4.
Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, et al. Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes. 2005;54:2320–7.
Yamaguchi GC, Nemezio K, Schulz ML, Natali J, Cesar JE, Riani LA, et al. Kinetics of muscle carnosine decay after β-alanine supplementation: A 16-wk washout study. Med Sci Sport Exerc. 2021;53:1079–88.
Gallo-Villegas J, Aristizabal JC, Estrada M, Valbuena LH, Narvaez-Sanchez R, Osorio J, et al. Efficacy of high-intensity, low-volume interval training compared to continuous aerobic training on insulin resistance, skeletal muscle structure and function in adults with metabolic syndrome: Study protocol for a randomized controlled clinical trial. Trials. 2018;19:144.
Gallo-Villegas J, Castro-Valencia LA, Pérez L, Restrepo D, Guerrero O, Cardona S, et al. Efficacy of high-intensity interval- or continuous aerobic-training on insulin resistance and muscle function in adults with metabolic syndrome: a clinical trial. Eur J Appl Physiol. 2022;122:331–44.
Painelli V, Nemezio KM, Pinto AJ, Franchi M, Andrade I, Riani LA, et al. High-intensity interval training augments muscle carnosine in the absence of dietary beta-alanine intake. Med Sci Sport Exerc. 2018;50:2242–52.
Jansen JFA, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–32.
Hoult DI. The principle of reciprocity. J Magn Reson. 2011;213:344–6.
Haase A, Hanicke W, Frahm J. The influence of experimental parameters in surface-coil NMR. J Magn Reson. 1984;56:401–12.
Skiba P, Fulford J, Clarke D, Vanhatalo A, Jones A. Intramuscular determinants of the ability to recover work capacity above critical power. Eur J Appl Physiol. 2015;115:703–13.
Suzuki Y, Ito O, Mukai N, Takahashi H, Takamatsu K. High level of skeletal muscle carnosine contributes to the latter half of exercise performance during 30-s maximal cycle ergometer sprinting. Jpn J Physiol. 2002;52:199–205.
Flanagan M. The behavior of quantization spectra as a function of signal-to-noise ratio. TDA Prog Rep. 1991;42–107:155–68.
Poullet J, Sima D, Van Huffel S. MRS signal quantitation: a review of time- and frequency-domain methods. J Magn Reson. 2008;195:134–44.
Everaert I, Mooyaart A, Baguet A, Zutinic A, Baelde H, Achten E, et al. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids. 2011;40:1221–9.
Wang X, Salibi N, Fayad L, Barker P. Proton magnetic resonance spectroscopy of skeletal muscle: a comparison of two quantitation techniques. J Magn Reson. 2014;243:81–4.
Acknowledgements
The authors want to thank to the volunteers and Funding
Funding
CODI, 2565, Juan C Calderón. Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS)-Coldeportes, Convocatoria 626-2014, project 111562638757 from 2014, Juan C Calderón. Universidad de Antioquia, 13041, Juan C Calderón. Fundación Banco de la República, 4339, Juan C Calderón.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures involving human participants in this study were in accordance with the ethical standards of the 1993 Resolution number 8430 by the National Ministry of Health of Colombia, the Bioethics Committee of the University of Antioquia (minutes 013 from September 2013) and IPS Universitaria (minutes 97 from June 2016) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vega, G., Ricaurte, G., Estrada-Castrillón, M. et al. In vivo absolute quantification of carnosine in the vastus lateralis muscle with 1H MRS using a surface coil and water as internal reference. Skeletal Radiol 52, 157–165 (2023). https://doi.org/10.1007/s00256-022-04149-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-022-04149-8