Abstract
Diabetes mellitus is a chronic metabolic disease, posing a considerable threat to global public health. Treating systemic comorbidities has been one of the greatest clinical challenges in the management of diabetes. Diabetic bladder dysfunction, characterized by detrusor overactivity during the early stage of the disease and detrusor underactivity during the late stage, is a common urological complication of diabetes. Oxidative stress is thought to trigger hyperglycaemia-dependent tissue damage in multiple organs; thus, a growing body of literature has suggested a possible link between functional changes in urothelium, muscle and the corresponding innervations. Improved understanding of the mechanisms of oxidative stress could lead to the development of novel therapeutics to restore the redox equilibrium and scavenge excessive free radicals to normalize bladder function in patients with diabetes.
Key points
-
Oxidative stress, characterized by either excessive reactive oxygen species production or disturbed antioxidant regulation, occurs in biological systems in normal and pathological conditions.
-
In physiological conditions, reactive oxygen species have a crucial role in cell metabolism, proliferation, differentiation, inflammatory response and preservation of mitochondrial function.
-
The balance between oxidants and antioxidants is maintained through several intertwined mechanisms that could be vulnerable to hyperglycaemia, leading to dysregulated oxidative stress and diabetic complications.
-
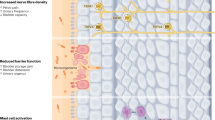
Diabetic bladder dysfunction (DBD) can manifest with functional abnormalities varying over time, characterized by a sensory urgency in the early stage, and an impaired sensation of bladder fullness and impaired bladder emptying in the late stage.
-
Hyperglycaemia-induced oxidative stress leading to DBD might also be responsible for impaired contractility of smooth muscle, altered sensory response of urothelium, and slowed conduction of lower urinary tract innervation.
-
In-depth research on dysregulated mechanisms of oxidative stress will facilitate the development of novel treatments targeting deficient pathways to mitigate DBD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 42, S13–S28 (2019).
Shamseddeen, H., Getty, J. Z., Hamdallah, I. N. & Ali, M. R. Epidemiology and economic impact of obesity and type 2 diabetes. Surg. Clin. North. Am. 91, 1163–1172 (2011).
Saeedi, P. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 157, 107843 (2019).
DeFronzo, R. A. et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 1, 15019 (2015).
Huang, E. S. Management of diabetes mellitus in older people with comorbidities. BMJ 353, i2200 (2016).
Yang, W. et al. Estimating costs of diabetes complications in people <65 years in the U.S. using panel data. J. Diabetes Complicat. 34, 107735 (2020).
Giri, B. et al. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: an update on glucose toxicity. Biomed. Pharmacother. 107, 306–328 (2018).
Paul, S., Ali, A. & Katare, R. Molecular complexities underlying the vascular complications of diabetes mellitus — a comprehensive review. J. Diabetes Complicat. 34, 107613 (2020).
Gomez, C. S., Kanagarajah, P. & Gousse, A. E. Bladder dysfunction in patients with diabetes. Curr. Urol. Rep. 12, 419–426 (2011).
Gandhi, J., Dagur, G., Warren, K., Smith, N. L. & Khan, S. A. Genitourinary complications of diabetes mellitus: an overview of pathogenesis, evaluation, and management. Curr. Diabetes Rev. 13, 498–518 (2017).
Brown, J. S. et al. Urologic complications of diabetes. Diabetes Care 28, 177–185 (2005).
Arrellano-Valdez, F., Urrutia-Osorio, M., Arroyo, C. & Soto-Vega, E. A comprehensive review of urologic complications in patients with diabetes. SpringerPlus 3, 549 (2014).
Nirmal, J. et al. Functional and molecular characterization of hyposensitive underactive bladder tissue and urine in streptozotocin-induced diabetic rat. PLoS One 9, e102644 (2014).
Palleschi, G. et al. Overactive bladder in diabetes mellitus patients: a questionnaire-based observational investigation. World J. Urol. 32, 1021–1025 (2014).
Fayyad, A. M., Hill, S. R. & Jones, G. Prevalence and risk factors for bothersome lower urinary tract symptoms in women with diabetes mellitus from hospital-based diabetes clinic. Int. Urogynecol. J. Pelvic Floor. Dysfunct. 20, 1339–1344 (2009).
Qaseem, A. et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann. Intern. Med. 168, 569–576 (2018).
Wang, R., Lefevre, R., Hacker, M. R. & Golen, T. H. Diabetes, glycemic control, and urinary incontinence in women. Female Pelvic Med. Reconstr. Surg. 21, 293–297 (2015).
Liu, N. et al. Relationship between blood glucose and hemoglobin A1c Levels and urinary incontinence in women. Int. J. Gen. Med. 14, 4105–4116 (2021).
Gulur, D. M., Mevcha, A. M. & Drake, M. J. Nocturia as a manifestation of systemic disease. BJU Int. 107, 702–713 (2011).
Yoshimura, N., Chancellor, M. B., Andersson, K. E. & Christ, G. J. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 95, 733–738 (2005).
Fedele, D. Therapy insight: sexual and bladder dysfunction associated with diabetes mellitus. Nat. Clin. Pract. Urol. 2, 282–290 (2005). quiz 309.
Fowler, C. J., Griffiths, D. & de Groat, W. C. The neural control of micturition. Nat. Rev. Neurosci. 9, 453–466 (2008).
Lightner, D. J., Gomelsky, A., Souter, L. & Vasavada, S. P. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment 2019. J. Urol. 202, 558–563 (2019).
Lee, W. C., Wu, C. C., Wu, H. P. & Tai, T. Y. Lower urinary tract symptoms and uroflowmetry in women with type 2 diabetes mellitus with and without bladder dysfunction. Urology 69, 685–690 (2007).
Frimodt-Moller, C. Diabetic cystopathy. A review of the urodynamic and clinical features of neurogenic bladder dysfunction in diabetes mellitus. Dan. Med. Bull. 25, 49–60 (1978).
Kaplan, S. A., Te, A. E. & Blaivas, J. G. Urodynamic findings in patients with diabetic cystopathy. J. Urol. 153, 342–344 (1995).
Harding, C. K. et al. EAU guidelines on management of non-neurogenic female lower urinary tract symptoms (LUTS). (EAU, 2021).
Abraham, N. & Goldman, H. B. An update on the pharmacotherapy for lower urinary tract dysfunction. Expert. Opin. Pharmacother. 16, 79–93 (2015).
Kuo, Y.-C. & Kuo, H.-C. Botulinum toxin injection for lower urinary tract dysfunction. Int. J. Urol. 20, 40–55 (2012).
Bartley, J., Gilleran, J. & Peters, K. Neuromodulation for overactive bladder. Nat. Rev. Urol. 10, 513–521 (2013).
Chancellor, M. B. & Kaufman, J. Case for pharmacotherapy development for underactive bladder. Urology 72, 966–967 (2008).
Osman, N. I. & Chapple, C. R. Are there pharmacotherapeutic options for underactive bladder? Eur. Urol. Focus. 4, 6–7 (2018).
Barendrecht, M. M., Oelke, M., Laguna, M. P. & Michel, M. C. Is the use of parasympathomimetics for treating an underactive urinary bladder evidence-based? BJU Int. 99, 749–752 (2007).
Yuan, Z., Tang, Z., He, C. & Tang, W. Diabetic cystopathy: a review. J. Diabetes 7, 442–447 (2015).
Andersson, K. E. Oxidative stress and its possible relation to lower urinary tract functional pathology. BJU Int. 121, 527–533 (2018).
Pizzino, G. et al. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 8416763 (2017).
Lushchak, V. I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 224, 164–175 (2014).
Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4, 180–183 (2015).
Zuo, L., Zhou, T., Pannell, B. K., Ziegler, A. C. & Best, T. M. Biological and physiological role of reactive oxygen species-the good, the bad and the ugly. Acta Physiol. 214, 329–348 (2015).
Zhang, L. et al. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 26, 101284 (2019).
Brand, M. D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 45, 466–472 (2010).
Roberge, S. et al. TNF-α-mediated caspase-8 activation induces ROS production and TRPM2 activation in adult ventricular myocytes. Cardiovasc. Res. 103, 90–99 (2014).
Clauzure, M. et al. Disruption of interleukin-1β autocrine signaling rescues complex I activity and improves ROS levels in immortalized epithelial cells with impaired cystic fibrosis transmembrane conductance regulator (CFTR) function. PLoS One 9, e99257 (2014).
Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K. & Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 (1995).
Woo, C. H. et al. Tumor necrosis factor-α generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J. Biol. Chem. 275, 32357–32362 (2000).
Amir Aslani, B. & Ghobadi, S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 146, 163–173 (2016).
He, L. et al. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 44, 532–553 (2017).
Yoshioka, J. Thioredoxin superfamily and its effects on cardiac physiology and pathology. Compr. Physiol. 5, 513–530 (2015).
Benhar, M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic. Biol. Med. 127, 160–164 (2018).
Arevalo, J. A. & Vazquez-Medina, J. P. The role of peroxiredoxin 6 in cell signaling. Antioxidants https://doi.org/10.3390/antiox7120172 (2018).
Ledgerwood, E. C., Marshall, J. W. & Weijman, J. F. The role of peroxiredoxin 1 in redox sensing and transducing. Arch. Biochem. Biophys. 617, 60–67 (2017).
Huang, J. Q., Zhou, J. C., Wu, Y. Y., Ren, F. Z. & Lei, X. G. Role of glutathione peroxidase 1 in glucose and lipid metabolism-related diseases. Free Radic. Biol. Med. 127, 108–115 (2018).
Gervasoni, B. D., Khairallah, G. N., O’Hair, R. A. & Wille, U. The role of peroxyl radicals in polyester degradation — a mass spectrometric product and kinetic study using the distonic radical ion approach. Phys. Chem. Chem. Phys. 17, 9212–9221 (2015).
Kanti Das, T., Wati, M. R. & Fatima-Shad, K. Oxidative stress gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s disease. Arch. Neurosci. https://doi.org/10.5812/archneurosci.20078 (2014).
Janciauskiene, S. The beneficial effects of antioxidants in health and diseases. Chronic Obstr. Pulm. Dis. 7, 182–202 (2020).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Zhang, J. et al. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 4350965 (2016).
Morgan, M. J. & Liu, Z. G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21, 103–115 (2011).
Navarro-Yepes, J. et al. Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid. Redox Signal. 21, 66–85 (2014).
Zmijewski, J. W. et al. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 285, 33154–33164 (2010).
Tang, J. Y. et al. Oxidative stress-modulating drugs have preferential anticancer effects — involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 58, 109–117 (2019).
Newsholme, P., Cruzat, V. F., Keane, K. N., Carlessi, R. & de Bittencourt, P. I. Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 473, 4527–4550 (2016).
Volpe, C. M. O., Villar-Delfino, P. H., Dos Anjos, P. M. F. & Nogueira-Machado, J. A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell death Dis. 9, 119 (2018).
Ye, J. et al. The role of autophagy in pro-inflammatory responses of microglia activation via mitochondrial reactive oxygen species in vitro. J. Neurochem. 142, 215–230 (2017).
Cairns, R. A., Harris, I. S. & Mak, T. W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 11, 85–95 (2011).
Moloney, J. N. & Cotter, T. G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 80, 50–64 (2018).
Chavda, V. et al. Molecular mechanisms of oxidative stress in stroke and cancer. Brain Disord. 5, 100029 (2021).
Dizdaroglu, M. & Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 46, 382–419 (2012).
Pravalika, K. et al. Myeloperoxidase and neurological disorder: a crosstalk. ACS Chem. Neurosci. 9, 421–430 (2018).
Liu, C. et al. Sulforaphane ameliorates bladder dysfunction through activation of the Nrf2-ARE pathway in a rat model of partial bladder outlet obstruction. Oxid. Med. Cell. Longev. 2016, 7598294 (2016).
Ener, K. et al. Evaluation of oxidative stress status and antioxidant capacity in patients with painful bladder syndrome/interstitial cystitis: preliminary results of a randomised study. Int. Urol. Nephrol. 47, 1297–1302 (2015).
Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53, 401–426 (2013).
Tonelli, C., Chio, I. I. C. & Tuveson, D. A. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 29, 1727–1745 (2018).
Suzuki, T. & Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 88, 93–100 (2015).
Tebay, L. E. et al. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 88, 108–146 (2015).
Raghunath, A. et al. Antioxidant response elements: discovery, classes, regulation and potential applications. Redox Biol. 17, 297–314 (2018).
Zhang, H., Davies, K. J. A. & Forman, H. J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 88, 314–336 (2015).
Chung, S. S., Ho, E. C., Lam, K. S. & Chung, S. K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 14, S233–S236 (2003).
Obrosova, I. G. Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid. Redox Signal. 7, 1543–1552 (2005).
Gabbay, K. H. The sorbitol pathway and the complications of diabetes. N. Engl. J. Med. 288, 831–836 (1973).
Ighodaro, O. M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 108, 656–662 (2018).
Wu, J., Jin, Z., Zheng, H. & Yan, L. J. Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. 9, 145–153 (2016).
Bertero, E. & Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 122, 1460–1478 (2018).
Gordeeva, A. V., Zvyagilskaya, R. A. & Labas, Y. A. Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry 68, 1077–1080 (2003).
Adam-Vizi, V. & Starkov, A. A. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J. Alzheimers Dis. 20 (Suppl 2), S413–S426 (2010).
Kim, A. N., Jeon, W. K., Lee, J. J. & Kim, B. C. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic. Biol. Med. 49, 323–331 (2010).
Batoko, H., Veljanovski, V. & Jurkiewicz, P. Enigmatic translocator protein (TSPO) and cellular stress regulation. Trends Biochem. Sci. 40, 497–503 (2015).
Ilkan, Z. & Akar, F. G. The mitochondrial translocator protein and the emerging link between oxidative stress and arrhythmias in the diabetic heart. Front. Physiol. 9, 1518 (2018).
Zorov, D. B., Filburn, C. R., Klotz, L. O., Zweier, J. L. & Sollott, S. J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192, 1001–1014 (2000).
Gliozzi, M. et al. Role of TSPO/VDAC1 upregulation and matrix metalloproteinase-2 localization in the dysfunctional myocardium of hyperglycaemic rats. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21207432 (2020).
Giatti, S. et al. Neuroprotective effects of a ligand of translocator protein-18 kDa (Ro5-4864) in experimental diabetic neuropathy. Neuroscience 164, 520–529 (2009).
Schwartz, S. S. et al. A unified pathophysiological construct of diabetes and its complications. Trends Endocrinol. Metab. 28, 645–655 (2017).
Kang, Q. & Yang, C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 37, 101799 (2020).
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107, 1058–1070 (2010).
Turkmen, K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: the four horsemen of the apocalypse. Int. Urol. Nephrol. 49, 837–844 (2017).
Dewanjee, S. et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 833, 472–523 (2018).
Singh, R., Kishore, L. & Kaur, N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol. Res. 80, 21–35 (2014).
Tang, W. H., Martin, K. A. & Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 3, 87 (2012).
Berthiaume, J. M., Kurdys, J. G., Muntean, D. M. & Rosca, M. G. Mitochondrial NAD+/NADH redox state and diabetic cardiomyopathy. Antioxid. Redox Signal. 30, 375–398 (2019).
Andres-Hernando, A., Johnson, R. J. & Lanaspa, M. A. Endogenous fructose production: what do we know and how relevant is it. Curr. Opin. Clin. Nutr. Metab. Care 22, 289–294 (2019).
DiNicolantonio, J. J., O’Keefe, J. H. & Lucan, S. C. Added fructose: a principal driver of type 2 diabetes mellitus and its consequences. Mayo Clin. Proc. 90, 372–381 (2015).
Basciano, H., Federico, L. & Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2, 5 (2005).
Akamine, T., Kusunose, N., Matsunaga, N., Koyanagi, S. & Ohdo, S. Accumulation of sorbitol in the sciatic nerve modulates circadian properties of diabetes-induced neuropathic pain hypersensitivity in a diabetic mouse model. Biochem. Biophys. Res. Commun. 503, 181–187 (2018).
Wu, M. Y., Yiang, G. T., Lai, T. T. & Li, C. J. The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid. Med. Cell. Longev. 2018, 3420187 (2018).
Lorenzi, M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp. Diabetes Res. 2007, 61038 (2007).
Hashimoto, Y. et al. Polyol pathway and diabetic nephropathy revisited: early tubular cell changes and glomerulopathy in diabetic mice overexpressing human aldose reductase. J. Diabetes Invest. 2, 111–122 (2011).
He, J. et al. The aldose reductase inhibitor epalrestat exerts nephritic protection on diabetic nephropathy in db/db mice through metabolic modulation. Acta Pharmacol. Sin. 40, 86–97 (2019).
Ramirez, M. A. & Borja, N. L. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy 28, 646–655 (2008).
Reiser, J. & Altintas, M. M. Podocytes. F1000Res https://doi.org/10.12688/f1000research.7255.1 (2016).
Kiyono, Y., Kajiyama, S., Fujiwara, H., Kanegawa, N. & Saji, H. Influence of the polyol pathway on norepinephrine transporter reduction in diabetic cardiac sympathetic nerves: implications for heterogeneous accumulation of MIBG. Eur. J. Nucl. Med. Mol. Imaging 32, 438–442 (2005).
D’Souza, D. R. et al. Hyperglycemia regulates RUNX2 activation and cellular wound healing through the aldose reductase polyol pathway. J. Biol. Chem. 284, 17947–17955 (2009).
Cheng, J. T. & Tong, Y. C. Alterations of nerve-growth factor and p75(NTR) expressions in urinary bladder of fructose-fed obese rats. Neurosci. Lett. 441, 25–28 (2008).
Tong, Y. C. & Cheng, J. T. Aldose reductase inhibitor ONO-2235 restores the alterations of bladder nerve growth factor and neurotrophin receptor p75 genetic expression in streptozotocin induced diabetic rats. J. Urol. 178, 2203–2207 (2007).
Hanna-Mitchell, A. T. et al. Impact of diabetes mellitus on bladder uroepithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R84–R93 (2013).
Mannikarottu, A. S., Changolkar, A. K., Disanto, M. E., Wein, A. J. & Chacko, S. Over expression of smooth muscle thin filament associated proteins in the bladder wall of diabetics. J. Urol. 174, 360–364 (2005).
Changolkar, A. K. et al. Diabetes induced decrease in detrusor smooth muscle force is associated with oxidative stress and overactivity of aldose reductase. J. Urol. 173, 309–313 (2005).
Wang, Y., Deng, G. G. & Davies, K. P. Novel insights into development of diabetic bladder disorder provided by metabolomic analysis of the rat nondiabetic and diabetic detrusor and urothelial layer. Am. J. Physiol. Endocrinol. Metab. 311, E471–E479 (2016).
Nowotny, K., Jung, T., Hohn, A., Weber, D. & Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5, 194–222 (2015).
Brings, S. et al. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18050984 (2017).
Moldogazieva, N. T., Mokhosoev, I. M., Mel’nikova, T. I., Porozov, Y. B. & Terentiev, A. A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid. Med. Cell. Longev. 2019, 3085756 (2019).
Gali, A. et al. Correlation between advanced glycation end-products, lower urinary tract symptoms and bladder dysfunctions in patients with type 2 diabetes mellitus. Low. Urin. Tract. Symptoms 9, 15–20 (2017).
Mancini, V. et al. Is coexistent overactive-underactive bladder (with or without detrusor overactivity and underactivity) a real clinical syndrome? ICI-RS 2019. Neurourol. Urodyn. 39(Suppl 3), S50–S59 (2020).
Kanwar, M. & Kowluru, R. A. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes 58, 227–234 (2009).
Kizub, I. V., Klymenko, K. I. & Soloviev, A. I. Protein kinase C in enhanced vascular tone in diabetes mellitus. Int. J. Cardiol. 174, 230–242 (2014).
Loscalzo, J. The identification of nitric oxide as endothelium-derived relaxing factor. Circulation Res. 113, 100–103 (2013).
Vanhoutte, P. M., Shimokawa, H., Feletou, M. & Tang, E. H. Endothelial dysfunction and vascular disease — a 30th anniversary update. Acta Physiol. 219, 22–96 (2017).
Nobe, K., Yamazaki, T., Tsumita, N., Hashimoto, T. & Honda, K. Glucose-dependent enhancement of diabetic bladder contraction is associated with a rho kinase-regulated protein kinase C pathway. J. Pharmacol. Exp. Ther. 328, 940–950 (2009).
Baumel-Alterzon, S., Katz, L. S., Brill, G., Garcia-Ocana, A. & Scott, D. K. Nrf2: the master and captain of β cell fate. Trends Endocrinol. Metab. 32, 7–19 (2021).
Tan, Y. et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 60, 625–633 (2011).
Whitmarsh, A. J. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim. Biophys. Acta 1773, 1285–1298 (2007).
Vessieres, E. et al. COX-2-derived prostanoids and oxidative stress additionally reduce endothelium-mediated relaxation in old type 2 diabetic rats. PLoS One 8, e68217 (2013).
Ganesh Yerra, V., Negi, G., Sharma, S. S. & Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 1, 394–397 (2013).
Horn, S. et al. Research resource: a dual proteomic approach identifies regulated islet proteins during beta-cell mass expansion in vivo. Mol. Endocrinol. 30, 133–143 (2016).
Salazar-Petres, E. R. & Sferruzzi-Perri, A. N. Pregnancy-induced changes in beta-cell function: what are the key players? J. Physiol. 600, 1089–1117 (2022).
Yagishita, Y., Uruno, A., Chartoumpekis, D. V., Kensler, T. W. & Yamamoto, M. Nrf2 represses the onset of type 1 diabetes in non-obese diabetic mice. J. Endocrinol. https://doi.org/10.1530/JOE-18-0355 (2019).
Kumar, A. et al. Activation of Nrf2 is required for normal and ChREBPα-augmented glucose-stimulated beta-cell proliferation. Diabetes 67, 1561–1575 (2018).
Li, L. et al. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 59, 152774 (2019).
Yu, H. et al. Rg1 protects H9C2 cells from high glucose-/palmitate-induced injury via activation of AKT/GSK-3β/Nrf2 pathway. J. Cell. Mol. Med. 24, 8194–8205 (2020).
Huang, K., Gao, X. & Wei, W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-β1 expressions in rat glomerular mesangial cells. Exp. Cell Res. 361, 63–72 (2017).
Ha, U. S. et al. Protective effect of cyanidin-3-O-beta-D-glucopyranoside fraction from mulberry fruit pigment against oxidative damage in streptozotocin-induced diabetic rat bladder. Neurourol. Urodyn. 32, 493–499 (2013).
Elrashidy, R. A. & Liu, G. Long-term diabetes causes molecular alterations related to fibrosis and apoptosis in rat urinary bladder. Exp. Mol. Pathol. 111, 104304 (2019).
Wang, J. et al. IR-61 improves voiding function via mitochondrial protection in diabetic rats. Front. Pharmacol. 12, 608637 (2021).
Cao, N., Gu, B., Gotoh, D. & Yoshimura, N. Time-dependent changes of urethral function in diabetes mellitus: a review. Int. Neurourol. J. 23, 91–99 (2019).
Daneshgari, F., Liu, G., Birder, L., Hanna-Mitchell, A. T. & Chacko, S. Diabetic bladder dysfunction: current translational knowledge. J. Urol. 182, S18–S26 (2009).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001).
Golbidi, S. & Laher, I. Bladder dysfunction in diabetes mellitus. Front. Pharmacol. https://doi.org/10.3389/fphar.2010.00136 (2010).
Abler, L. L. & Vezina, C. M. Respiratory physiology & neurobiology links between lower urinary tract symptoms, intermittent hypoxia and diabetes: causes or cures. Respir. Physiol. Neurobiol. https://doi.org/10.1016/j.resp.2017.09.009 (2017).
Kirschner-Hermanns, R. et al. Does diabetes mellitus-induced bladder remodeling affect lower urinary tract function? ICI-RS 2011. Neurourol. Urodyn. 31, 359–364 (2012).
Daneshgari, F. et al. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1728–R1735 (2006).
Daneshgari, F., Liu, G. & Imrey, P. B. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J. Urol. 176, 380–386 (2006).
Liu, G., Lin, Y. H., Yamada, Y. & Daneshgari, F. External urethral sphincter activity in diabetic rats. Neurourol. Urodyn. 27, 429–434 (2008).
Yono, M., Latifpour, J., Yoshida, M. & Ueda, S. Age-related alterations in the biochemical and functional properties of the bladder in type 2 diabetic GK rats. J. Recept. Signal. Transduct. Res. 25, 147–157 (2005).
Gasbarro, G. et al. Voiding function in obese and type 2 diabetic female rats. Am. J. Physiol. Renal Physiol. 298, F72–F77 (2010).
Kendig, D. M., Ets, H. K. & Moreland, R. S. Effect of type II diabetes on male rat bladder contractility. Am. J. Physiol. Renal Physiol. 310, F909–F922 (2016).
Kim, A. K., Hamadani, C., Zeidel, M. L. & Hill, W. G. Urological complications of obesity and diabetes in males and females of three mouse models: temporal manifestations. Am. J. Physiol. Renal Physiol. 318, F160–F174 (2020).
Klee, N. S., Moreland, R. S. & Kendig, D. M. Detrusor contractility to parasympathetic mediators is differentially altered in the compensated and decompensated states of diabetic bladder dysfunction. Am. J. Physiol. Renal Physiol. 317, F388–F398 (2019).
Aizawa, N., Homma, Y. & Igawa, Y. Characteristics of lower urinary tract dysfunction and bladder afferent nerve properties in type 2 diabetic Goto-Kakizaki rats. J. Urol. 189, 1580–1587 (2013).
Sullivan, C. J. et al. Microarray analysis reveals novel gene expression changes associated with erectile dysfunction in diabetic rats. Physiol. Genomics 23, 192–205 (2005).
Hipp, J. D. et al. Using gene chips to identify organ-specific, smooth muscle responses to experimental diabetes: potential applications to urological diseases. BJU Int. 99, 418–430 (2007).
Kanika, N. D. et al. Oxidative stress status accompanying diabetic bladder cystopathy results in the activation of protein degradation pathways. BJU Int. 107, 1676–1684 (2011).
Yohannes, E., Chang, J., Christ, G. J., Davies, K. P. & Chance, M. R. Proteomics analysis identifies molecular targets related to diabetes mellitus-associated bladder dysfunction. Mol. Cell Proteom. 7, 1270–1285 (2008).
Tomechko, S. E. et al. Tissue specific dysregulated protein subnetworks in type 2 diabetic bladder urothelium and detrusor muscle. Mol. Cell Proteom. 14, 635–645 (2015).
Poladia, D. P. & Bauer, J. A. Oxidant driven signaling pathways during diabetes: role of Rac1 and modulation of protein kinase activity in mouse urinary bladder. Biochimie 86, 543–551 (2004).
Li, W. J. & Oh, S. J. Diabetic cystopathy is associated with PARP/JNK/mitochondrial apoptotic pathway-mediated bladder apoptosis. Neurourol. Urodyn. 29, 1332–1337 (2010).
Kaneto, H. et al. Oxidative stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Curr. Mol. Med. 7, 674–686 (2007).
Beshay, E. & Carrier, S. Oxidative stress plays a role in diabetes-induced bladder dysfunction in a rat model. Urology 64, 1062–1067 (2004).
Elrashidy, R. A. et al. Smooth muscle-specific deletion of MnSOD exacerbates diabetes-induced bladder dysfunction in mice. Am. J. Physiol. Renal Physiol. 317, F906–F912 (2019).
Lee, W. C., Chien, C. T., Yu, H. J. & Lee, S. W. Bladder dysfunction in rats with metabolic syndrome induced by long-term fructose feeding. J. Urol. 179, 2470–2476 (2008).
Birder, L. A. et al. How does the urothelium affect bladder function in health and disease? ICI-RS 2011. Neurourol. Urodyn. 31, 293–299 (2012).
Merrill, L., Gonzalez, E. J., Girard, B. M. & Vizzard, M. A. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat. Rev. Urol. 13, 193–204 (2016).
Birder, L. A. & de Groat, W. C. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat. Clin. Pract. Urol. 4, 46–54 (2007).
Dalghi, M. G., Montalbetti, N., Carattino, M. D. & Apodaca, G. The urothelium: life in a liquid environment. Physiol. Rev. 100, 1621–1705 (2020).
Hurst, R. E. et al. In the absence of overt urothelial damage, chondroitinase ABC digestion of the GAG layer increases bladder permeability in ovariectomized female rats. Am. J. Physiol. Renal Physiol. 310, F1074–F1080 (2016).
Coccheri, S. Approaches to prevention of cardiovascular complications and events in diabetes mellitus. Drugs 67, 997–1026 (2007).
Pinna, C., Bolego, C. & Puglisi, L. Effect of substance P and capsaicin on urinary bladder of diabetic rats and the role of the epithelium. Eur. J. Pharmacol. 271, 151–158 (1994).
Pinna, C., Caratozzolo, O. & Puglisi, L. A possible role for urinary bladder epithelium in bradykinin-induced contraction in diabetic rats. Eur. J. Pharmacol. 214, 143–148 (1992).
Pinna, C., Zanardo, R. & Puglisi, L. Prostaglandin-release impairment in the bladder epithelium of streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 388, 267–273 (2000).
Tong, Y. C., Cheng, J. T. & Hsu, C. T. Alterations of M2-muscarinic receptor protein and mRNA expression in the urothelium and muscle layer of the streptozotocin-induced diabetic rat urinary bladder. Neurosci. Lett. 406, 216–221 (2006).
Cheng, J. T., Yu, B. C. & Tong, Y. C. Changes of M3-muscarinic receptor protein and mRNA expressions in the bladder urothelium and muscle layer of streptozotocin-induced diabetic rats. Neurosci. Lett. 423, 1–5 (2007).
Wang, C. C. & Kuo, H. C. Urothelial Dysfunction and chronic inflammation in diabetic patients with overactive bladder. Low. Urin. Tract. Symptoms 9, 151–156 (2017).
Poladia, D. P. & Bauer, J. A. Early cell-specific changes in nitric oxide synthases, reactive nitrogen species formation, and ubiquitinylation during diabetes-related bladder remodeling. Diabetes Metab. Res. Rev. 19, 313–319 (2003).
Deli, G., Bosnyak, E., Pusch, G., Komoly, S. & Feher, G. Diabetic neuropathies: diagnosis and management. Neuroendocrinology 98, 267–280 (2013).
Burakgazi, A. Z., Alsowaity, B., Burakgazi, Z. A., Unal, D. & Kelly, J. J. Bladder dysfunction in peripheral neuropathies. Muscle Nerve 45, 2–8 (2012).
Van Poppel, H., Stessens, R., Van Damme, B., Carton, H. & Baert, L. Diabetic cystopathy: neuropathological examination of urinary bladder biopsies. Eur. Urol. 15, 128–131 (1988).
Lee, W. C., Wu, H. P., Tai, T. Y., Yu, H. J. & Chiang, P. H. Investigation of urodynamic characteristics and bladder sensory function in the early stages of diabetic bladder dysfunction in women with type 2 diabetes. J. Urol. 181, 198–203 (2009).
Mitsui, T. et al. Vesicourethral function in diabetic patients: association of abnormal nerve conduction velocity with vesicourethral dysfunction. Neurourol. Urodyn. 18, 639–645 (1999).
Melman, A. et al. Longitudinal studies of time-dependent changes in both bladder and erectile function after streptozotocin-induced diabetes in Fischer 344 male rats. BJU Int. 104, 1292–1300 (2009).
Liu, G. & Daneshgari, F. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am. J. Physiol. Renal Physiol. 288, F1220–F1226 (2005).
Blaha, I. et al. Bladder dysfunction in an obese Zucker rat: the role of TRPA1 channels, oxidative stress, and hydrogen sulfide. Oxid. Med. Cell. Longev. 2019, 5641645 (2019).
Ochodnicky, P., Cruz, C. D., Yoshimura, N. & Michel, M. C. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol. Urodyn. 30, 1227–1241 (2011).
Song, Q. X., Chermansky, C. J., Birder, L. A., Li, L. & Damaser, M. S. Brain-derived neurotrophic factor in urinary continence and incontinence. Nat. Rev. Urol. 11, 579–588 (2014).
Steinbacher, B. C. Jr & Nadelhaft, I. Increased levels of nerve growth factor in the urinary bladder and hypertrophy of dorsal root ganglion neurons in the diabetic rat. Brain Res. 782, 255–260 (1998).
Sasaki, K. et al. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J. Urol. 168, 1259–1264 (2002).
Tong, Y. C. & Cheng, J. T. Changes in bladder nerve-growth factor and p75 genetic expression in streptozotocin-induced diabetic rats. BJU Int. 96, 1392–1396 (2005).
Nykjaer, A. & Willnow, T. E. Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci. 35, 261–270 (2012).
Barker, P. A. p75NTR is positively promiscuous: novel partners and new insights. Neuron 42, 529–533 (2004).
Mossa, A. H. et al. Antagonism of proNGF or its receptor p75NTR reverses remodelling and improves bladder function in a mouse model of diabetic voiding dysfunction. Diabetologia 63, 1932–1946 (2020).
Goins, W. F. et al. Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J. Urol. 165, 1748–1754 (2001).
Goss, J. R. et al. Herpes simplex-mediated gene transfer of nerve growth factor protects against peripheral neuropathy in streptozotocin-induced diabetes in the mouse. Diabetes 51, 2227–2232 (2002).
Sasaki, K. et al. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes 53, 2723–2730 (2004).
Azadzoi, K. M., Yalla, S. V. & Siroky, M. B. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J. Urol. 178, 710–715 (2007).
Li, B., Lang, N. & Cheng, Z. F. Serum levels of brain-derived neurotrophic factor are associated with diabetes risk, complications, and obesity: a cohort study from Chinese patients with type 2 diabetes. Mol. Neurobiol. 53, 5492–5499 (2016).
He, M. & Wang, J. Decreased serum brain-derived neurotrophic factor in Chinese patients with type 2 diabetes mellitus. Acta Biochim. Biophys. Sin. 46, 426–427 (2014).
Xue, J. et al. Caffeine improves bladder function in diabetic rats via a neuroprotective effect. Exp. Ther. Med. 21, 501 (2021).
Sun, Z. et al. NGF protects against oxygen and glucose deprivation-induced oxidative stress and apoptosis by up-regulation of HO-1 through MEK/ERK pathway. Neurosci. Lett. 641, 8–14 (2017).
Li, R. et al. NGF attenuates high glucose-induced ER stress, preventing Schwann cell apoptosis by activating the PI3K/Akt/GSK3β and ERK1/2 pathways. Neurochem. Res. 42, 3005–3018 (2017).
Qi, G. et al. Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct. 8, 4421–4432 (2017).
Behl, T. & Kotwani, A. Downregulated brain-derived neurotrophic factor-induced oxidative stress in the pathophysiology of diabetic retinopathy. Can. J. Diabetes 41, 241–246 (2017).
Yamanaka, M. et al. Protective effect of brain-derived neurotrophic factor on pancreatic islets in obese diabetic mice. Metabolism 55, 1286–1292 (2006).
Tsounapi, P., Honda, M., Hikita, K., Sofikitis, N. & Takenaka, A. Oxidative stress alterations in the bladder of a short-period type 2 diabetes rat model: antioxidant treatment can be beneficial for the bladder. In Vivo 33, 1819–1826 (2019).
Lin, C. F. et al. Sulforaphane improves voiding function via the preserving mitochondrial function in diabetic rats. J. Formos. Med. Assoc. 119, 1422–1430 (2020).
Zhang, B. et al. Grape seed proanthocyanidin extract alleviates urethral dysfunction in diabetic rats through modulating the NO-cGMP pathway. Exp. Ther. Med. 15, 1053–1061 (2018).
Pergola, P. E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365, 327–336 (2011).
Himmelfarb, J. & Tuttle, K. R. New therapies for diabetic kidney disease. N. Engl. J. Med. 369, 2549–2550 (2013).
Zhang, P. et al. Oxidative stress and diabetes: antioxidative strategies. Front. Med. 14, 583–600 (2020).
Hybertson, B. M., Gao, B., Bose, S. K. & McCord, J. M. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Asp. Med. 32, 234–246 (2011).
Bouayed, J. & Bohn, T. Exogenous antioxidants — double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 3, 228–237 (2010).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. Q.X.S., M.S.D., Y.S. contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks A. Dobberfuhl and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Sensory urgency
-
Increased perceived bladder sensation during filling, with a low bladder capacity and a short voiding duration.
- Daytime frequency
-
Micturition occurring more frequently during waking hours than previously deemed normal by the patient (defined by the International Urogynecological Association/International Continence Society).
- Pressure flow urodynamic testing
-
Refers to the voiding part during a urodynamic test, in which bladder pressure is measured simultaneously with the urinary flow.
- Clean intermittent catheterization
-
A method of draining the urine out of the bladder using a thin, soft tube, which is removed immediately once the bladder is emptied.
- Timed voiding
-
To void at fixed time intervals, instead of relying on the sensation of bladder fullness.
- Double voiding
-
To pass urine more than once during each void with the purpose of reducing residual volume.
- Antioxidant response element
-
(ARE). Transcription regulators acting with nuclear factor erythroid 2-related factor 2 to restore redox homeostasis and to activate cytoprotection in response to oxidative stress.
- Streptozotocin (STZ)-induced rat model
-
An animal model of type 1 diabetes induced by intravenous injection of streptozotocin to cause pancreatic islet β cell destruction.
- International Prostatic Symptom Score
-
A questionnaire containing seven items to assess the severity of lower urinary tract symptoms in male patients.
- Threshold pressure
-
The pressure recorded just before the onset of voiding during cystometry.
- Cystometric contraction pressure
-
Calculated by subtracting threshold pressure from the maximum pressure recorded during micturition.
- Swollen mitochondria
-
A hallmark of mitochondrial dysfunction and cell death, which can be characterized by the opening of permeability transition pores.
- Metabolome
-
The complete collection of low-molecular weight metabolites, participating in the metabolic reactions to maintain cell physiological functions.
- Sortilin
-
A transmembrane protein that forms a receptor complex with p75 neurotrophin receptor, binding with a precursor of nerve growth factor at the cell surface.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, QX., Sun, Y., Deng, K. et al. Potential role of oxidative stress in the pathogenesis of diabetic bladder dysfunction. Nat Rev Urol 19, 581–596 (2022). https://doi.org/10.1038/s41585-022-00621-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00621-1
This article is cited by
-
Cellular zinc metabolism and zinc signaling: from biological functions to diseases and therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Predictive value of clinical risk factors for bladder dysfunction in Syrian patients with type 2 diabetes mellitus
Scientific Reports (2024)
-
Diabetes and the risk of bladder cancer subtypes in men and women: results from the Netherlands Cohort Study
European Journal of Epidemiology (2024)