Abstract
Purpose
This case–control study investigated the long-term evolution of multidrug-resistant bacteria (MDRB) over a 5-year period associated with the use of selective oropharyngeal decontamination (SOD) in the intensive care unit (ICU). In addition, effects on health care-associated infections and ICU mortality were analysed.
Methods
We investigated patients undergoing mechanical ventilation > 48 h in 11 adult ICUs located at 3 campuses of a university hospital. Administrative, clinical, and microbiological data which were routinely recorded electronically served as the basis. We analysed differences in the rates and incidence densities (ID, cases per 1000 patient-days) of MDRB associated with SOD use in all patients and stratified by patient origin (outpatient or inpatient). After propensity score matching, health-care infections and ICU mortality were compared.
Results
5034 patients were eligible for the study. 1694 patients were not given SOD. There were no differences in the incidence density of MDRB when SOD was used, except for more vancomycin-resistant Enterococcus faecium (0.72/1000 days vs. 0.31/1000 days, p < 0.01), and fewer ESBL-producing Klebsiella pneumoniae (0.22/1000 days vs. 0.56/1000 days, p < 0.01). After propensity score matching, SOD was associated with lower incidence rates of ventilator-associated pneumonia and death in the ICU but not with ICU-acquired bacteremia or urinary tract infection.
Conclusions
Comparisons of the ICU-acquired MDRB over a 5-year period revealed no differences in incidence density, except for lower rate of ESBL-producing Klebsiella pneumoniae and higher rate of vancomycin-resistant Enterococcus faecium with SOD. Incidence rates of ventilator-associated pneumonia and death in the ICU were lower in patients receiving SOD.
Similar content being viewed by others
In this case–control study in mechanically ventilated patients (> 48 h), no differences in the incidence density of multidrug-resistant bacteria were observed, except for a lower rate of Klebsiella pneumoniae with extended beta-lactamase and a higher rate of vancomycin-resistant Enterococcus faecium with selective oral decontamination. According to propensity score matching, ventilator-associated pneumonia and ICU deaths occurred less frequently with selective oral decontamination |
Introduction
In critically ill patients undergoing mechanical ventilation, the use of selective decontamination of the oropharynx (SOD) and the digestive tract without or with systemic antibiotics (SDD) still remains controversial [1,2,3,4,5]. The main argument against its use is the possible ecological impact on antibiotic resistance. Although recent publications in intensive care units (ICUs) with low and highly resistant microorganisms show that prolonged use of SOD or SDD has no overall ecological impact, both regimens are not widely used in ICUs in Europe [6,7,8,9,10,11].
In Germany, a country with medium rates of multidrug-resistant bacteria (MDRB), SOD is used in 24.1% of all adult ICUs (unpublished data from the ONTAI study) [12]. Even in large hospitals, ICU directors disagree on its use resulting in different preventive strategies. At Munich University Hospital (LMU Klinikum), a large-scale hospital at two campuses and an affiliated cardiac clinic, SOD without systemic antibiotics is in clinical use in some multidisciplinary ICUs for more than 3 decades [13, 14]. Close surveillance and strict infection control have not shown any differences in the resistance rates of bacterial pathogens over the years. However, the increasing prevalence of MDRB in German ICUs carries the risk of higher selection under SOD in mechanically ventilated critically ill patients and thus poses a threat [15].
The aim of this study was to analyse the emergence of MDRB associated with SOD over a period of 5 years in mechanically ventilated patients. In particular, it should be determined which MDRB emerged during and after the use of SOD in the ICU with focus on extended-spectrum beta-lactamases (ESBL) expressing Enterobacterales and carbapenemase-producing Gram-negative bacteria (GNB) [5]. Possible interactions through the administration of systemic antibiotics were also analysed.
Methods
This is a case–control study of the long-term effects of SOD on ecological safety in 11 adult intensive care units of a 2000-bed hospital of the Ludwig-Maximilians-University of Munich (LMU Klinikum). The intensive care units at LMU Hospital are spread across three locations within the city limits, on two campuses and in a cooperating cardiac clinic. The study protocol was approved by the Ethical Committee (EC) of the LMU in December 2016 (Projekt Nr: 733-16). The EC waived the requirement for informed patient consent because of anonymous data processing after completion of data sampling and eligibility.
The study protocol was prepared and discussed with an interdisciplinary group of infectiologists, microbiologists, pharmacists, and intensive care physicians (iKUM Group, Infektiologie am Klinikum der Universität München). Data were collected and analysed by a computer scientist trained in health informatics and qualified in medicine. The adherence to preventive measures and procedures to control ventilator-associated pneumonia were investigated in all participating ICUs according to the recommendations given by a national group of experts of the Robert-Koch-Institute in Berlin, Germany [16].
Since 1987, SOD has been a routine procedure for prevention of ventilator-associated pneumonia in some ICUs of the LMU hospital based on a previous study of our institution [13]. Over the decades, only SOD (without IV antibiotics) was performed in routine clinical practice, except during the period of participation in a prospective randomized controlled trial (RCT), where intravenous ciprofloxacin was additionally administered [14]. From 2011, a hospital-wide written SOP was introduced and SOD, when performed, was done in a standardised manner by nursing staff. Compliance with the SOP was part of a quality assurance measure conducted in parallel with this analysis. The SOD solution was prepared by the pharmacy department. Each 80 ml bottle contained 9.5 mg per ml colistin sulphate and 13.6 mg per ml gentamicin sulphate in a sodium hydrogen sulphate solution. A single dose consisted of 10 ml, of which 5 ml was administered into the oral cavity and 5 ml into the gastric tube, which was clamped for 30 min. The SOD was administered every 6 h. Commercially available solutions of nystatin or amphotericin B (in transplanted or immunosuppressed patients) were administered every 6 h in between. Prior to administration, either oral hygiene was performed or at least the oral cavity and nasopharynx was suctioned. SOD was administered until the tracheal tube was removed. In patients with a tracheal cannula via a tracheostoma, in transplanted patients and in immunosuppressed patients, SOD was administered until discharge from the ICU.
For the objective of this analysis, all directors of the 11 ICUs of the hospital were contacted and informed about the purpose of the study. They provided detailed information on preventive measures and procedures to control ventilator-associated pneumonia and on the routine use or non-use of SOD in mechanically ventilated patients during the study period (January 2011 to December 2016). Intensive care units that did not use SOD served as control.
The primary objective of this exploratory analysis was to detect differences in the evolution of multidrug-resistant bacteria (MDRB) associated with the use of selective oropharyngeal decontamination in critically ill patients undergoing mechanical ventilation > 48 h. We analysed all patients admitted to the ICU and used all routine surveillance samples taken in the patients under study at admission and during the stay in the ICU.
MDRBs that developed during the stay in the ICU were considered, and the respective incidence density and resistance rate of MDRBs were calculated. We used the definition of the German Hospital Infection Surveillance for Intensive Care Units (ITS-KISS) to distinguish MDRBs that were already present on admission to the ICU or acquired during the ICU stay [17]. From day 3 onward in the ICU, if emerging MDRBs were cultured in any sample taken, they were considered acquired during the ICU stay. MDRBs isolated within the first two days in the ICU or up to 14 days prior to ICU admission have been documented but were not considered associated with the use of SOD. In addition, we carried out subgroup analyses in patients who were admitted directly to the ICU from the community and in patients who had already been hospitalised outside the ICU for > 48 h. Secondary endpoints were rates of MDRB in all cultures taken at ICU admission (in the first 2 days) and incidence density of MDRB in total (MDRB at admission and ICU-acquired).
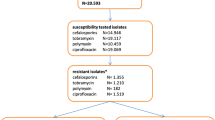
Overall, the ecological impact on resistance was assessed in the participating ICUs, including surveillance and clinical samples. Surveillance endpoints were the rates and incidence densities of MDRB such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VRE), extended-spectrum beta-lactamase (ESBL) producing Escherichia coli and Klebsiella pneumoniae, fluoroquinolone-resistant Escherichia coli, carbapenem-resistant Klebsiella pneumoniae, Enterobacter cloacae, Serratia marcescens, Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Acinetobacter baumannii. Resistance were analysed in total, in all cultures taken at admission to the ICU (on day 1 or 2 of stay on the ICU) and during the stay in the ICU (on day 3 or later of stay on the ICU) according to the criteria of the German Nosocomial Infection Surveillance System (KISS) [18].
The results of all microbiological examinations carried out during the study period were taken from an electronic database of the Department of Medical Microbiology and Hospital Hygiene (Max von Pettenkofer Institute, LMU Munich) and combined with electronic clinical data of the study patients. After data processing, the data were irreversibly anonymised. The incidence density (ID) under SOD was calculated by dividing the number of first incident isolation of multidrug-resistant pathogens by the cumulative sum of days of length of stay in the ICU related to 1,000 patient-days. Repeat cultures confirming a previous finding of MDRB with the same resistance profile during the ICU stay were not counted for the analysis.
In addition, in order to detect a possible selection pressure under SOD, the development of the incidence density of MDRB, which differed between the groups, was analysed and compared over the 5 years of the study period. The overall antimicrobial use was assessed by recording all administered antibiotics (except from SOD) by the defined daily doses (DDD) for each participating ICU. The antibiotic use density was calculated as follows: (antibiotic use in g/DDD in g) × 100 patient-days.
The secondary objective of this study was to analyse health care-associated infections (HAI) such as ventilator-associated pneumonia, nosocomial blood stream infection and nosocomial urinary tract infections during the intervention with SOD [19]. For the diagnosis of ventilator-associated pneumonia (VAP), any documented pneumonia that occurred during the period under mechanical ventilation was used [20]. For the diagnosis of bacteremia, every blood culture in which a pathological pathogen could be cultivated was counted. In case of Staphylococcus epidermidis, at least two blood cultures taken from different puncture sites had to be positive to be counted. For urinary tract infections, we counted any catheter-associated urinary tract infection in which > 105 CFU per millilitre were detected in the urine collected from the urinary catheter.
For the propensity score analysis, the variables were matched for age, gender, duration of mechanical ventilation, sepsis severity score [21], first SAPS II score [22], length of stay (LOS) in the ICU and for type of admission. Finally, we assessed observed incidence death rates before and after propensity score matching.
Statistical analysis
We used MedCalc® Statistical Software version 20 (MedCalc Software Ltd, Ostend, Belgium) and the open source statistical software R (version 3.6.0, Vienna, Austria) for statistical analysis. The incidence rate was compared between the groups as the ratio of MDRB first isolates and the total number of days in the ICU. Repeated comparisons of each MDRB over the years were corrected according to Bonferroni–Holms. To obtain two highly similar groups for comparison of health-care infections and death in the ICU, propensity score matching was performed considering 7 variables such as age, sex, duration of mechanical ventilation, sepsis severity, Simplified Acute Physiology Score (SAPS) II at ICU admission, length of ICU stay and type of ICU admission (surgical or non-surgical). Propensity scores were matched using nearest-neighbour matching to achieve lowest standardised mean differences of the seven variables. After matching, scores were tested for normality. Incidence rates were compared as the ratio between the number of events observed and the total number of days at risk. Trends over years between the groups were tested with repeated measures analysis of variance. p values < 0.05 were considered significant.
Results
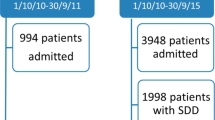
Of the 11 ICUs participating in this analysis, 7 ICUs used SOD routinely in mechanically ventilated patients and 3 ICUs did not use any SOD. One ICU used SOD only in selected patients at high risk for VAP. Due to this inconsistent use of SOD, 478 ventilated patients in this ICU were excluded from case–control study (Fig. 1). Additional preventive measures and procedures to control ventilator-associated pneumonia were followed in the ten remaining ICUs in the same way as documented in a separate quality control analysis.
Flow chart of the patients in this study. Incidence density was assessed in 3340 patients with SOD and in 1694 patients without SOD (1 >), in 1280 patients with SOD and 1076 patients without SOD admitted to the ICU from the community (2 >), and in 1077 patients with SOD and 372 patients without SOD who were already in hospital but not in the ICU 48 h before admission to the ICU (3 >). For comparison of health care-associated infections and mortality in the ICU, 1694 patients without SOD were compared with corresponding patients with SOD according to a 1:1 propensity score matching (4 >)
Between January 2012 and December 2016, 12,172 consecutive adult (≥ 18 years) critically ill patients undergoing mechanical ventilation (MV) were identified from the hospital electronic databases. 7138 patients were excluded because of MV ≤ 48 h (6252 patients), use of SOD only in selected patients in 1 ICU (478 patients) and additional 408 patients because of incomplete electronic datasets (see Fig. 1).
During the study period of 5 years, 5034 patients were eligible for the study from whom a total of 143.842 microbiological test results were available (respiratory samples n = 26.957, blood cultures n = 20.719, urine sample n = 19.655; rectal swabs n = 4.605, other samples n = 71.906). 1694 patients did not receive topical SOD and 3340 patients did (Fig. 1, 1>). Out of these patients, 1280 patients with SOD and 1076 patients without SOD were admitted from the community to the ICUs (Fig. 1, 2>), whereas 1077 and 372 patients were already in the hospital but outside the ICU > 48 h prior to admission (Fig. 1, 3 >).
Characteristics of all included patients with mechanical ventilation (> 48 h) are given in Table 1. Large standardised mean differences (SMD) between the two groups (SMD > ± 0.3) were found for the type of ICU admission, with more surgical and transplant patients in the SOD group and more medical patients in the non-SOD group. Length of stay in the ICU was also longer in the SOD group. Other differences were small (SMD ≤ ± 0.3). After propensity score matching two comparable groups of 1694 patients were generated with high agreement in age, gender, duration of mechanical ventilation, sepsis severity score, SAPS II score, LOS in the ICU, and type of admission (SMD < ± 0.03, see Table 2).
Ecological effects on MDRB
During the 5-year study period, the rates and incidence densities of all ICU-acquired MDRB such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VRE), extended-spectrum beta-lactamase (ESBL) producing Escherichia coli and Klebsiella pneumoniae, fluoroquinolone-resistant Escherichia coli, carbapenem-resistant Klebsiella pneumoniae, Enterobacter cloacae, Serratia marcescens, Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Acinetobacter baumannii of all included patients did not differ between the two groups with the exception of vancomycin-resistant Enterococcus faecium (VRE) being significantly increased, and Klebsiella pneumonia ESBL being significantly decreased in the group undergoing SOD (Table 3). Incidence densities were about twice as high in the SOD group for VRE (0.72/1000 days vs. 0.31/1000 days, p < 0.01), and about half as high for ESBL-producing Klebsiella pneumoniae (0.22/1000 days vs. 0.56/1000 days, p < 0.01). MRSA rates tended to be lower in the SOD group (0.19/1000 days vs. 0.37/1000 days, p = 0.06). Analysis of patients admitted to the ICU directly from the community also found that VRE rates develop more frequently in patients with SOD (Table 4). In patients who were treated in the hospital for more than 48 h prior to admission to the ICU, we found a lower incidence rate of Klebsiella pneumoniae ESBL in the group treated with SOD (Table 5). The evolution of the ICU-acquired MDRB over the 5 years revealed no trend of MDRB selection found to be different between the groups in the analysis (Fig. 2). The incidence rate of all MDRB in samples taken at admission and during the stay in the ICU differed for MRSA, VRE, and ESBL-producing Klebsiella pneumoniae between the groups (Tab. S1). On admission, patients in the SOD group had a higher rate of Pseudomonas aeruginosa.
Consumption of antibacterial drugs in all patients (n = 12,172) treated in intensive care units during the entire study period is shown in the supplement (including patients treated for less than 48 h or excluded for other reasons, Fig. S1). Overall, there was a significantly higher consumption of antibiotics administered intravenously in the ICUs with SOD. The cumulative DDD per 100 patient-days were 147 (95% CI 138-155) in the SOD group vs. 125 (95% CI 115-135) the group without SOD. ICUs with SOD used more carbapenems, 2nd-generation cephalosporins, glycopeptides, and other antibiotics, whereas ICUs without SOD used more 3rd-generation cephalosporins, penicillins with beta-lactamase inhibitors, and fluoroquinolones.
Effect of SOD on health care-associated infections
Propensity analysis of nosocomial infections (HAI) revealed lower incidence rates and densities of ventilator-associated pneumonia but not bacteremia or urinary tract infections in the group receiving SOD (Table 6). The number needed to prevent a VAP was 29. The incidence densities found in the studied patients were higher than reported by national surveillance.
Effect of SOD on death in the ICU
The incidence rate of death in the ICU was significantly lower in the SOD group. After 1:1 propensity score matching for demographic characteristics, sepsis severity, severity of illness, length of mechanical ventilation, LOS in the ICU and type of admission, mortality in the ICU was 28% in the group receiving SOD and 30% in the group not receiving SOD (Table 7).
Discussion
This case–control study of 5034 mechanically ventilated critically ill patients over a 5-year period found an incidence density of all MRDB equal to or lower than that reported for German ICUs in 2015 [15]. With SOD, there were no statistically significant differences in the incidence densities of ICU-acquired MDRB under study, with the exception of vancomycin-resistant Enterococcus faecium (VRE), which was more common with SOD, and extended beta-lactamase Klebsiella pneumoniae, which was less common with SOD. There was no trend towards an increased selection of these MDRBs over the years observed. The propensity score analysis of health care-associated infections found that SOD was associated with less ventilation-associated pneumonia, but not with bacteremia or urinary tract infections acquired in the intensive care unit. Incidence rate of death in the ICU was lower in the SOD group.
Three limitations of this case–control study should be noted. First, there was already a difference in the rates of MDRB isolates at admission to the ICU, which can be attributed to the specifics of patients under study. Although we excluded MDRB isolates found in the first 48 h after ICU admission, this could influence the analysis. Second, many characteristics and disease severity were similar in the two groups, but there were more surgical patients in the SOD group and more medical patients in the non-SOD group (Table 1). The surgical patients included also patients after solid organ transplantation such as heart, lung, and liver transplantation. In this group of patients, the rates of Pseudomonas aeruginosa were found more frequently already at admission to the ICU (see Table S2 in the supplement). The increased rate of Pseudomonas aeruginosa on admission may be related to patients with cystic fibrosis admitted prior and after lung transplantation. Although this particular patient cohort was more common in the SOD group, no higher rate of ICU-acquired Pseudomonas could be observed. Third, it was not possible to stratify the included patients according to the systemic antibiotics they received, since only the total antibiotic consumption of all patients treated in the intensive care units during the study period was available.
The increased occurrence of VRE under SOD may be related to the fact that there were more surgical patients in the SOD group. Many isolates with E. faecium in this study were found in swabs from rectum, wounds and abscesses. This may have led to the fact that E. faecium and thus VRE were found more frequently in the group with SOD during the stay in the ICU. In the analysis of antibiotic selection pressure over the study period of 5 years, our results did not support the assumption that selection pressure increased under SOD. It is noteworthy, that during the study period, the prevalence of VRE increased in German hospitals, particularly in southern regions of Germany accounting for 11 to 26% of all isolates [15, 23]. This was attributed, in part, to an extensive use of fluoroquinolones in outpatients in the corresponding areas of Germany [15, 23]. Both the trend over the years and the distribution of isolates at the end of this study suggest against an association with the use of SOD.
An new finding from this study is that ESBL-producing Klebsiella pneumoniae was more common in ICU patients not treated with SOD. When comparing the antibiotics used systemically during ICU stay, there was no difference in the indication for antibiotic therapy, that could explain this finding. SOD may be specifically successful in preventing colonisation and subsequent infection with ESBL-producing Klebsiella pneumoniae.
We found an increased total consumption of antibacterial drugs in intensive care units with SOD. This can be attributed, among other things, to the large number of postoperative surgical patients who spent only a day or two in the ICU and were not included in this study. A large proportion of these patients received antibiotics as perioperative antibiotic prophylaxis.
The propensity score analysis of health care-associated infections showed that ventilator-associated pneumonia occurred less frequently with SOD than in patients without SOD. This is consistent with the results of controlled studies on this prophylactic measure [14, 24, 25]. The extent to which the additional systemic administration of cefotaxime for 4 days would have further reduced this incidence, as recently described [25], cannot be assessed on the basis of our data. Blood stream infections did not differ between the groups, which is consistent with findings of a large European trial [26].
The propensity score analysis also showed lower death rate in the SOD group. Both groups were equally severely ill and also well matched for LOS in the ICU and type of admission. Therefore, the observed lower ICU mortality in surgical patients compared to non-surgical patients can, therefore, be ruled out as a cause [2, 27].
Conclusion
In this case–control study of mechanically ventilated adult patients (> 48 h), we found that the use of SOD was not associated with the development of antibiotic resistance in a set of ICUs with medium rates of MDR bacteria. According to propensity score matching, a recognised approach for causal inference in observational data, the use of SOD was associated with a lower incidence rate of ventilator-associated pneumonia and death in the ICU. Even though encouraging, these findings could not be extrapolated to intensive care units with high rates of MDR bacteria. Future studies are still required for these settings.
References
de Smet AMGA, Kluytmans JAJW, Cooper BS, Mascini EM, Benus RFJ, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJM, Bernards AT, Kuijper EJ, Joore JCA, Leverstein-van Hall MA, Bindels AJGH, Jansz AR, Wesselink RMJ, de Jongh BM, Dennesen PJW, van Asselt GJ, te Velde LF, Frenay IHME, Kaasjager K, Bosch FH, van Iterson M, Thijsen SFT, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LPHJ, Sturm PDJ, Harinck HIJ, Voss A, Uijtendaal EV, Blok HEM, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJM (2009) Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360:20–31
Oostdijk EAN, Kesecioglu J, Schultz MJ, Visser CE, de Jonge E, van Essen EHR, Bernards AT, Purmer I, Brimicombe R, Bergmans D, van Tiel F, Bosch FH, Mascini E, van Griethuysen A, Bindels A, Jansz A, van Steveninck FAL, van der Zwet WC, Fijen JW, Thijsen S, de Jong R, Oudbier J, Raben A, van der Vorm E, Koeman M, Rothbarth P, Rijkeboer A, Gruteke P, Hart-Sweet H, Peerbooms P, Winsser LJ, van Elsacker-Niele AW, Demmendaal K, Brandenburg A, de Smet A, Bonten MJM (2014) Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA 312:1429–1437
Vincent J-L, Jacobs F (2011) Effect of selective decontamination on antibiotic resistance. Lancet Infect Dis 11:337–338
Silvestri L, van Saene HKF, Bion J (2018) Antipathy against SDD is justified: No. Intensive Care Med 44:1169–1173
Timsit JF, Bassetti M (2018) Antipathy against SDD is justified: Yes. Intensive Care Med 44:1165–1168
Buitinck S, Jansen R, Rijkenberg S, Wester JPJ, Bosman RJ, van der Meer NJM, van der Voort PHJ (2019) The ecological effects of selective decontamination of the digestive tract (SDD) on antimicrobial resistance: a 21-year longitudinal single-centre study. Crit Care 23:208
Llorens-Villar Y, Tusell F, Canut A, Barrasa H, Corral E, Martin A, Rodriguez-Gascon A (2019) Antibiotic susceptibility trend before and after long-term use of selective digestive decontamination: a 16 year ecological study. J Antimicrob Chemother 74:2289–2294
Cuthbertson BH (2018) Selective decontamination of the digestive tract in critical care: a teenage angst or coming of age issue? Crit Care 22:296
Cavalcanti AB, Lisboa T, Gales AC (2017) Is selective digestive decontamination useful for critically ill patients? Shock 47:52–57
Daneman N, Sarwar S, Fowler RA, Cuthbertson BH, Su DCSG (2013) Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 13:328–341
Sanchez-Ramirez C, Hipola-Escalada S, Cabrera-Santana M, Hernandez-Viera MA, Caipe-Balcazar L, Saavedra P, Artiles-Campelo F, Sangil-Monroy N, Lubbe-Vazquez CF, Ruiz-Santana S (2018) Long-term use of selective digestive decontamination in an ICU highly endemic for bacterial resistance. Crit Care 22:141
Liebchen U, Paal M, Scharf C, Schroeder I, Grabein B, Zander J, Siebers C, Zoller M (2020) The ONTAI study—a survey on antimicrobial dosing and the practice of therapeutic drug monitoring in German intensive care units. J Crit Care 60:260–266
Unertl K, Ruckdeschel G, Selbmann HK, Jensen U, Forst H, Lenhart FP, Peter K (1987) Prevention of colonization and respiratory infections in long-term ventilated patients by local antimicrobial prophylaxis. Intensive Care Med 13:106–113
Krueger WA, Lenhart FP, Neeser G, Ruckdeschel G, Schreckhase H, Eissner HJ, Forst H, Eckart J, Peter K, Unertl KE (2002) Influence of combined intravenous and topical antibiotic prophylaxis on the incidence of infections, organ dysfunctions, and mortality in critically ill surgical patients: a prospective, stratified, randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med 166:1029–1037
Remschmidt C, Schneider S, Meyer E, Schroeren-Boersch B, Gastmeier P, Schwab F (2017) Surveillance of Antibiotic Use and Resistance in Intensive Care Units (SARI). Dtsch Arztebl Int 114:858–865
KRINKO (2013) Prävention der nosokomialen beatmungsassoziierten Pneumonie. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 56:1578–1590
Gastmeier P, Behnke M, Breier AC, Piening B, Schwab F, Dettenkofer M, Geffers C (2012) Healthcare-associated infection rates: measuring and comparing. Experiences from the German National Nosocomial Infection Surveillance System (KISS) and from other surveillance systems. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 55:1363–1369
Gastmeier P, Behnke M, Breier AC, Piening B, Schwab F, Dettenkofer M, Geffers C (2012) Nosokomiale Infektionsraten: Messen und Vergleichen. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 55:1363–1369
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332
Papazian L, Klompas M, Luyt CE (2020) Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med 46:888–906
Ford DW, Goodwin AJ, Simpson AN, Johnson E, Nadig N, Simpson KN (2016) A severe sepsis mortality prediction model and score for use with administrative data. Crit Care Med 44:319–327
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA J Am Med Assoc 270:2957–2963
Markwart R, Willrich N, Haller S, Noll I, Koppe U, Werner G, Eckmanns T, Reuss A (2019) The rise in vancomycin-resistant Enterococcus faecium in Germany: data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob Resist Infect Control 8:147
D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E, Liberati A (2009) Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000022.pub3
Bos LD, Stips C, Schouten LR, van Vught LA, Wiewel MA, Wieske L, van Hooijdonk RT, Straat M, de Beer FM, Glas GJ, Visser CE, de Jonge E, Juffermans NP, Horn J, Schultz MJ (2017) Selective decontamination of the digestive tract halves the prevalence of ventilator-associated pneumonia compared to selective oral decontamination. Intensive Care Med 43:1535–1537
Wittekamp BH, Plantinga NL, Cooper BS, Lopez-Contreras J, Coll P, Mancebo J, Wise MP, Morgan MPG, Depuydt P, Boelens J, Dugernier T, Verbelen V, Jorens PG, Verbrugghe W, Malhotra-Kumar S, Damas P, Meex C, Leleu K, van den Abeele AM, Pimenta G, de Matos AF, Fernandez Mendez S, Vergara Gomez A, Tomic V, Sifrer F, Villarreal Tello E, Ruiz Ramos J, Aragao I, Santos C, Sperning RHM, Coppadoro P, Nardi G, Brun-Buisson C, Bonten MJM (2018) Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA 320:2087–2098
Oostdijk EAN, Kesecioglu J, Schultz MJ, Visser CE, de Jonge E, van Essen EHR, Bernards AT, Purmer I, Brimicombe R, Bergmans D, van Tiel F, Bosch FH, Mascini E, van Griethuysen A, Bindels A, Jansz A, van Steveninck FAL, van der Zwet WC, Fijen JW, Thijsen S, de Jong R, Oudbier J, Raben A, van der Vorm E, Koeman M, Rothbarth P, Rijkeboer A, Gruteke P, Hart H, Peerbooms P, Winsser LJ, van Elsacker-Niele AW, Demmendaal K, Brandenburg A, de Smet A, Bonten MJM (2017) Notice of Retraction and Replacement: Oostdijk et al. Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA 317(15):1583–1584
Acknowledgements
First and foremost, the authors would like to thank all the nurses in our intensive care units who, as a quality assurance survey showed, follow the SOP on the use of selective oral decontamination with high adherence. This dedicated work made this case–control study possible. We also thank the directors of the ICUs of LMU Hospital Munich, Germany, for providing details on patient management and prevention of ventilator-associated pneumonia as detailed in the doctoral thesis of R. Fritsche, LMU Munich 2019. Our special thanks go to M. Angstwurm, Medical Clinic 4, W. Hartl, Surgical Clinic, S. Kääb, Medical Clinic 1, A. Peraud, Neurosurgical Clinic, H.-W. Pfister, Neurological Clinic, and H.-J. Stemmler, Medical Clinic 3.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported by funds of the Department of Anesthesiology, Klinikum der Ludwig-Maximilians-Universität, München, Germany.
Author information
Authors and Affiliations
Contributions
Design, data acquisition, analysis, interpretation, drafting and revising the manuscript, and final approval: JB, BW, JJ, AW, RD, JB, LH, and BG; data acquisition, analysis, interpretation, drafting the manuscript, and final approval: WK, UL, SN, MZ, and SN; interpretation, drafting and revising the manuscript, and final approval: SF, MI, LN, TW, EK, and PM. BW and JB contributed equally to this work.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, B., Briegel, J., Krueger, W.A. et al. Ecological effects of selective oral decontamination on multidrug-resistance bacteria acquired in the intensive care unit: a case–control study over 5 years. Intensive Care Med 48, 1165–1175 (2022). https://doi.org/10.1007/s00134-022-06826-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-022-06826-7
Keywords
- Selective oral decontamination, selective digestive decontamination, antimicrobial resistance, intensive care units/statistics and numerical data
- Methicillin-resistant Staphylococcus aureus/drug effects
- Klebsiella pneumoniae/drug effects
- Polymyxins/drug effects
- Pseudomonas aeruginosa/drug effects
- Vancomycin/drug effects