Abstract
In northwestern Ethiopia, a conventional teff (Eragrostis tef (Zucc.) Trotter) monoculture was converted into a rotational agroforestry system (teff-Acacia agroforestry), which consists of the sequence i) teff-Acacia decurrens intercropping (1st year), ii) grass-A. decurrens silvopasture (2nd year), iii) A. decurrens plantation (3rd–4th year), and iv) on-site charcoal production from A. decurrens (end of 4th year). This study is the first one to comprehensively show how the agroforestry system affects soil organic carbon (SOC) and soil nutrients. Soil samples were collected from the teff-Acacia agroforestry and conventional teff fields at two different sites (site I and II) and they were used to determine soil pH, black carbon, SOC, soil total nitrogen (STN), extractable phosphorus (P), potassium (K), magnesium (Mg), sodium (Na), and calcium (Ca) contents. At site I (0–10 cm soil depth), charcoal production spots in teff-Acacia agroforestry had higher soil pH (20%), black carbon (164%), SOC (48%), P (687%), and K (788%) contents compared to outside charcoal production spots. Soil organic carbon and STN contents in the 1st and 2nd teff-Acacia agroforestry rotations were significantly higher (SOC: 112–169%, STN: 100–131%) than teff fields. At site II, SOC stocks (0–100 cm) in the 1st agroforestry rotation were not significantly different from teff fields. However, they were 159% and 244% greater in the 2nd and 3rd agroforestry rotations, respectively, compared to teff fields. Conversion of teff fields to teff-Acacia agroforestry for a 12-year period increased SOC stocks by 21 Mg C ha–1 per year. Our results demonstrated that locally adopted agroforestry practices can increase SOC and nutrients in the long term, thereby contributing to enhanced soil fertility and improved climate change mitigation strategies via carbon sequestration.
Similar content being viewed by others
1 Introduction
Agroforestry is any practice that purposefully integrates trees and shrubs into crop and animal farming systems (Jose et al. 2012). It improves various ecosystem services such as increased food production, improved soil fertility, and enhanced carbon sequestration and mitigation of greenhouse gas (GHG) emissions (Muchane et al. 2020; Corbeels et al. 2019). Various studies reported that agroforestry increases soil C and N (Muchane et al. 2020; Kuyah et al. 2019). In agroforestry systems, soil C and N originate from external inputs of organic matter (e.g., compost, manure) and inorganic fertilizer, and the internal cycling of C and N (e.g., nitrogen fixation, leaf litter, dead roots, nodules, and microbial necromass) (Muchane et al. 2020; Zhu et al. 2020; Corbeels et al. 2019; Isaac and Borden 2019). Most soil C and N are found in organic form as part of soil organic matter (SOM) (Weil and Brady 2017), and SOM protection inside aggregates is an important mechanism for C and N storage in soil (Muchane et al. 2020; Fonte et al. 2010). Global meta-analyses revealed that soil C sequestration rates in agroforestry systems ranged from 1.4 to 2.2 Mg C ha−1 yr−1 (Feliciano et al. 2018; Kim et al. 2016a). The annual SOC sequestration in agroforestry systems in sub-Saharan Africa (SSA) has been documented to amount up to 14 Mg C ha–1 yr–1 (0−100 cm; Corbeels et al. 2019). A global meta-analysis (Muchane et al. 2020) revealed that soil N stocks under agroforestry were 46% higher than in crop monocultures in the humid and sub-humid tropics. A meta-analysis of SSA studies (Kuyah et al. 2019) found that agroforestry increased soil N by 20%. In addition, various studies showed that agroforestry practices increase crop yields, which were attributed to various factors including improved soil C and N (Kuyah et al. 2019; Akinnifesi et al. 2010). A meta-analysis of field studies conducted in SSA (Sileshi et al. 2008) revealed that agroforestry increased maize yields by 89–318%. These results suggest that agroforestry could make a strong contribution to increasing soil C and N, which would potentially enhance food security and carbon sequestration.
The effect of biochar application on SOC in agro-ecosystems has been studied globally (Wang et al. 2020; Bai et al. 2019; Majumder et al. 2019). The studies commonly observed an increase in stabilized SOC (Wang et al. 2020; Bai et al. 2019). Global meta-analyses found that biochar amendment increased SOC content by 40% Schmidt et al. 2021; Bai et al. 2019; Liu et al. 2016). The increased SOC content could be mainly attributed to the high C content of biochar and its prevailing polycondensed aromatic structure (Glaser et al. 1998), which makes it more resistant to microbial degradation (Wang et al. 2016; Lorenz and Lal 2014). In addition, biochar amendment results in an increase in soil pH due to its alkaline nature and high pH buffering capacity (Kavitha et al. 2018; Dai et al. 2017) and overall positive effects on soil N and P availability (Ahmad et al. 2021; Glaser and Lehr 2019). In various regions (e.g., Amazon, Asia, and Africa), traditional charcoal production spots (e.g., traditional earth mound kilns) showed significantly higher soil C and nutrient contents compared to spots outside charcoal production (Coomes and Miltner 2017; Miltner and Coomes 2015). Amendment of charcoal debris increased soil pH, soil C, and nutrient contents (Berihun et al. 2017; Bayabil et al. 2015). These findings are in line with our understanding of the effect of biochar on soil properties and its mechanism (Kavitha et al. 2018; Dai et al. 2017).
In northwestern Ethiopia, local farmers have been converting conventional teff (Eragrostis tef (Zucc.) Trotter) fields to a unique, locally adopted agroforestry practice (Nigussie et al. 2017, 2020; Berihun et al. 2019), consisting of a sequence of i) teff—Acacia decurrens intercropping (year 1), ii) grass—A. decurrens silvopasture (year 2), iii) A. decurrens plantation (year 3 and 4), and iv) charcoal production from A. decurrens after 4 years and ploughing fields with the remaining charcoal debris for the next rotation, starting again with teff—A. decurrens intercropping (Fig. 1). Since soil in the area is degraded and acidic, local farmers do not obtain sufficient crop yield (Nigussie et al. 2017). To resolve this issue, they fallowed their land by planting A. decurrens and adopted the agroforestry practice (Nigussie et al. 2017, 2021; Wondie and Mekuria 2018). Local farmers selected A. decurrens since they can harvest the trees after a short period of 4 years and produce charcoal of high quality, which can be a source of energy or alternative income generation (Nigussie et al. 2020, 2021; Abeje et al. 2019). The new practice has been quickly transferred to nearby areas (Wondie and Mekuria 2018; Nigussie et al. 2017).
It is not uncommon for trees (e.g., Acacia abyssinica, Acacia tortilis, Acacia polyacantha, Acacia senegal, Acacia seyal, and Faidherbia albida) to be integrated into agricultural practices in Ethiopia. Previous studies identified various advantages and potentials of this practice for enhancing soil fertility and SOC sequestration (Terefe and Kim 2020; Birhane et al. 2019). However, how the sequential practices of different agroforestry elements in northwestern Ethiopia affect SOC and soil nutrient has not been well understood yet. Therefore, the main objectives of this study were to assess the effect of the sequential agroforestry practices on soil nutrients and SOC and to identify the potential of agroforestry
2 Material and methods
2.1 General description of the study area
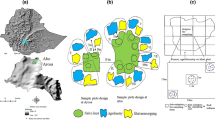
The study was conducted in Gafera kebele (the smallest administrative unit in Ethiopia) of Feggta Lekoma District, Awi Zone of Amhara Regional State, northwestern Ethiopia. The major soil type in the study area is a Nitisol (Elias 2016). Mean annual temperature and rainfall are 24.0 ± 2.0 °C and 1700 mm, respectively (Belayneh et al. 2020; Wondie and Mekuria 2018). The rainfall pattern of the area is bimodal, consisting of a short rainy season from mid-March to the end of May and a long rainy season from June to September/October. In the study area, main agricultural practices are continuous teff cropping and locally adopted agroforestry practice: sequential practice of teff—A. decurrens intercropping, grass—A. decurrens silvopasture, A. decurrens plantation and on-site charcoal production (hereafter teff-Acacia agroforestry) (Fig. 1). The different practices are described in the following.
2.1.1 Conventional teff cropping
In the study area, teff fields are tilled by horses at least four times to a depth of 15 to 20 cm from April to end of May/early June to smooth the land. Cattle manure (10–11 Mg ha–1) is spread before the fourth tilling, which starts 7 days after the third. During the fourth tilling, any weeds are removed from the field and the seedbed is prepared. Seven days after the fourth tilling, the field is trampled by cattle and horses before teff is sown. Di-ammonium phosphate (DAP) (50 kg ha–1; equivalent to 9 kg N ha–1 and 23 kg P ha–1) is then applied. Teff is harvested manually in October/November and threshed in December/January. The teff residues are transported to farms and used as animal feed or as mud house construction material. Livestock is allowed to enter the fields for grazing after teff is harvested.
2.1.2 Sequential practice of teff—acacia agroforestry
In the 1st year of teff-Acacia agroforestry, teff is cultivated as described above, but cattle manure and synthetic fertilizer are not applied. A. decurrens trees are planted by using seedlings with a spacing of 50 cm × 75 cm immediately after teff is sown. Teff is harvested in October/November (teff—A. decurrens intercropping period). After harvesting until the end of the 2nd year, local farmers let grasses grow beside A. decurrens (grass—A. decurrens silvopasture period). They cut and remove the grass for sale or to feed their own livestock. In the 3rd and 4th year, A. decurrens trees continuously grow without teff and grass cultivation (A. decurrens plantation period). At the end of the 4th year, the trees are harvested manually, and large roots are removed from the soil and brought to the boundary of the field. The harvested trees are de-branched and cut in pieces of approximately 30–50 cm length. The prepared A. decurrens logs are piled to heaps on the ground and covered with a small amount of teff straw and soil (thickness of 5–10 cm) for charcoal production (traditional earth-mound kilns in the area). Charcoal production spots are distributed throughout the fields (15 to 25 of charcoal production spots per hectare). The smoldering of A. decurrens logs is finished after 3 to 5 days (depending on the moisture content of the logs). The soil is then removed to retrieve the charcoal. After production of charcoal, around 8 kg of charcoal debris remains per charcoal production spot within a circle of about 3 m diameter. The charcoal debris is then incorporated into the soil (around 11 Mg ha-1 within the charcoal production spot) during tilling for the next teff–A. decurrens intercropping period of the teff-Acacia agroforestry rotation system.
2.2 Study sites and soil sampling
For this study, two different study sites (hereafter site I and site II) were selected. The sites and soil samplings are described below. Unless indicated otherwise, all results are given for 0–10 cm soil depth.
2.2.1 Site I
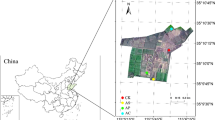
Study site I consisted of adjacently located conventional monoculture teff fields and teff-Acacia agroforestry fields of the 1st and 2nd rotation (11°05'19.9 " – 11°05' 24.9" N, 36°53'15.9" – 36°53' 24.9" E; elevation 2433–2435 m a. m. s. l.). Two different campaigns were conducted for 1) comparison of soil properties inside and outside charcoal production spots in teff-Acacia agroforestry fields, and 2) comparison of soil properties between teff monocropping and teff-Acacia agroforestry fields.
-
1)
Comparison of soil properties inside and outside charcoal production spots in teff-Acacia agroforestry
Three soil sampling spots were randomly selected and soil samples were collected inside and outside charcoal production spots in the 1st rotation of the teff-Acacia agroforestry system, where charcoal had been produced from harvested A. decurrens (4 years after conversion). The collected soil samples were analyzed for soil pH, black carbon, and total P, K, Mg, Na, Ca, SOC, and STN contents.
-
2)
Comparison of soil properties between teff and teff-Acacia agroforestry fields
Three soil sampling spots were randomly selected, and intact soil cores (0–10 cm depth and 7.2 cm diameter) were collected in each teff field and each teff-Acacia agroforestry field after the 1st rotation (after harvesting A. decurrens 4 years since conversion) and after the 2nd rotation (after harvesting A. decurrens at the beginning of the 9th year since conversion) field. The collected soil samples were analyzed for soil pH and total P, K, Mg, Na, Ca, SOC, and STN contents.
2.2.2 Site II
The study site II (11° 03' 29" to 11° 04' 01" N, 36° 54' 15" to 36° 57' 47" E; 2466 to 2525 m a. s. l.) consisted of adjacently located conventional teff monoculture fields and teff-Acacia agroforestry fields of the 1st, 2nd, and 3rd rotation (4, 8, and 12 years after conversion from teff monocropping fields). Soil texture, soil pH, bulk density, and SOC contents and stocks (0–10, 10–20, 20–40, 40–60, and 60–100 cm soil depth) were compared between teff fields and teff-Acacia agroforestry fields after harvest of teff and A. decurrens, respectively. For this purpose, soil samples were taken at four randomly selected soil sampling spots in each of the teff and teff-Acacia agroforestry fields. A 100-cm deep soil profile was dug at each sampling location, and intact soil core samples were collected with a core sampler (7.2 cm diameter, 10 cm height) at intervals of 10 cm along the whole 100 cm soil profile. Intact soil cores were used to determine soil bulk density. Extra soil samples were collected in the layers of 0–10, 10–20, 20–40, 40–60, and 60–100 cm and were analyzed for soil texture, soil pH, SOC contents, and stocks.
2.3 Soil analysis and calculations
Soil bulk density was determined by dividing oven-dry soil mass (dried at 105 °C for 72 hours) by the volume of soil cores (Grossman and Reinsch 2002). Soil subsamples were prepared by air-drying, crushing and thorough mixing. They were then sieved to 2 mm. Subsamples were used to determine soil pH (1:2 soil:H2O) using a pH meter (pH-8414, Wincom Company Ltd, China) and soil texture with the hydrometric method (Bouyoucos 1962). SOC content was determined with an elemental analyzer (Helios C/S Analyzer, Eltra GmbH, Haan, Germany) and STN content was determined with another elemental analyzer (Truspec CN, LECO, St. Joseph, MI, USA). Extractable P and K were determined with a continuous flow analyzer (Skalar San System, Skalar Analytical B.V., Breda, The Netherlands). Extractable Na and extractable Ca were determined with ICP-OES (725 E S, Agilent Technologies, Santa Clara, CA, USA) and Mg was determined with atomic absorption spectroscopy (AAnalyst 400, PerkinElmer, Waltham, MA, USA). Detailed information on soil analysis is provided in the Supplementary Information.
SOC stocks (Mg ha−1) for each sampled depth in the fields were calculated using the following equation (IPCC 2003) (1):
where C = SOC stocks (Mg ha−1) of a sample depth; z = thickness of a sample depth (cm); ρb = bulk density (g cm–3) of natural forest at sample depth; c = SOC content (g kg–1 soil) of sample depth; frag = % volume of coarse fragments/100, dimensionless. SOC stocks for the whole depth 0–100 cm were determined by summing up SOC stocks of each sample depth.
2.4 Charcoal and black carbon analysis
The pH of A. decurrens-derived charcoal was determined by treating 1 g of charcoal with 10 ml of distilled water and 1 M potassium chloride (KCl) solution. The extract was analyzed with a pH meter (ProfiLine pH/Cond 3320, WTW, Xylem Analytics, Germany). Total organic carbon (TOC) and total N of homogenized charcoal samples were measured using a EURO EA Elemental Analyzer (EuroVector, Hekatech, Germany) coupled via a Conflo III Interface to an isotope ratio mass spectrometer (IRMS; Finnigen Delta V Advantage, Thermo Scientific, Bremen, Germany). Extractable (with calcium acetate/lactate solution) and total P and K of charcoal were determined with a continuous flow analyzer (Skalar San System, Skalar Analytical B.V., Breda, The Netherlands). Black carbon in charcoal was analyzed using benzene polycarboxylic acid (BPCA) as a molecular marker (Glaser et al. 1998). During 65% nitric acid oxidation at 170 °C and high pressure for 8 h, polycondensed aromatic moieties of charcoal are converted to BPCA. After sample clean-up before and after this procedure and derivatization, BPCA can be identified and quantified using gas chromatography and flame ionization detection. Detailed information on charcoal and black carbon analysis is provided in the Supplementary Information.
2.5 Statistical analysis
The normality of all data distributions was first analyzed using the Shapiro–Wilk Normality Test (Shapiro and Wilk 1965). A t-test was used to evaluate the differences in mean values of soil pH, P, K, Mg, Na, Ca, black carbon, SOC, and STN contents and the C:N ratio inside and outside charcoal production spots in the 1st rotation of the teff-Acacia agroforestry system at site 1. When the standard assumptions of normality were violated, a Mann-Whitney rank sum test (Mann and Whitney 1947) was used. One-way analysis of variance (ANOVA) was used to evaluate the difference in mean values of soil pH, bulk density, sand, silt, and clay contents and stocks of SOC in teff fields and in fields of the 1st, 2nd, and 3rd rotation of the teff-Acacia agroforestry system at site 2. For the ANOVA, violation of assumptions of normal distribution (Shapiro–Wilk test), homoscedasticity (Durbin–Watson statistic), and constant variance (Spearman rank correlation) was checked (Motulsky and Christopoulos 2004). When the assumption was violated, the Kruskal-Wallis non-parametric analysis (Kruskal and Wallis 1952) was applied. To determine the relationship between the period of practicing teff-Acacia agroforestry and SOC stocks, Pearson correlation analysis was applied. All results were assessed at a 95% significance level. The statistical analyses were conducted using SigmaPlot Ver. 12.0 (Systat Software Inc., San Jose, CA, USA).
3 Results
3.1 Characteristics of A. decurrens-derived charcoal
The pH of A. decurrens-derived charcoal was 7.9 (pHH2O) and 7.6 (pHKCl). The black carbon content of charcoal was 228 ± 3 g kg–1, corresponding to 284 ± 3 g kg–1 TOC, with a dominance of the highly condensed aromatic moieties (47% benzene hexacarboxylic acid, 29% benzene pentacarboxylic acid, 19% benzene tetracarboxylic acids, 6% benzene tricarboxylic acids). Total N content of charcoal was less than the detection limit of 2 mg N kg–1. Total and extractable P and K contents of charcoal were 38 ± 14 and 9.9 ± 0.5 g P kg–1 and 380 ± 60 and 233 ± 4 g K kg–1, respectively.
3.2 Soil carbon and nutrients inside and outside charcoal production spots in teff-Acacia agroforestry (Site I)
At 0–10 cm soil depth, soil pH, P, K, black carbon, and SOC inside charcoal production spots in the 1st rotation of the teff-Acacia agroforestry system were significantly (all p < 0.05) higher (20%, 687%, 788%, 164%, and 48%, respectively) than those outside charcoal production spots (Table 1). Black carbon composition was comparable in all investigated soils (33% benzene hexacarboxylic acid, 33% benzene pentacarboxylic acid, 26% benzene tetracarboxylic acids, 7% benzene tricarboxylic acids). However, the contribution of highly condensed aromatic moieties was smaller compared to the pure Acacia charcoal (33% vs. 46% benzene hexacarboxylic acid). There was no significant difference in STN, Mg, Na, Ca, and C:N ratio inside and outside charcoal production spots (Table 1).
3.3 Soil carbon and nutrients in teff fields and teff-Acacia agroforestry (Site I)
At 0–10 cm soil depth, SOC contents were significantly higher in the 1st rotation (112% higher) and 2nd rotation of the teff-Acacia agroforestry system (169% higher) compared to teff monocropping fields (all p < 0.05) (Table 2). Soil total nitrogen contents were significantly higher in the 1st rotation (100% higher) and 2nd rotation of the teff-Acacia agroforestry system (131% higher) compared to conventional teff fields (all p < 0.05) (Table 2). However, there was no significant difference in SOC and STN contents between the 1st and 2nd teff-Acacia agroforestry rotation (Table 2). Soil pH and P, K, Mg, Na, and Ca contents were not significantly different across the sites (Table 2).
3.4 Soil texture, pH, and bulk density in teff fields and teff-Acacia agroforestry (Site II)
Throughout the whole soil profile (0–100 cm), there was no significant difference in soil texture fractions (sand, silt and clay) across the sites (Table 3). Across the sites (0–100 cm), sand, silt, and clay fractions ranged between 15–40.5, 25.5–68.5, and 16.5–39%, respectively (Table 3). For sand, the fractions significantly increased with increasing soil depth in the 1st and 2nd rotation of the teff-Acacia agroforestry system and the teff fields (all p < 0.05). For clay, the fractions significantly increased with increasing soil depth in the 2nd rotation and the teff fields (all p < 0.05).
At 0–60 cm soil depth, there was no significant difference in soil pH across the sites (Table 3). At the 60–100 cm soil depth interval, soil pH was not significantly different between the teff fields and the 1st and 3rd teff-Acacia agroforestry rotation, whereas soil pH in the 2nd teff-Acacia agroforestry rotation was significantly lower than in the teff fields (p = 0.021).
At the 0–60 cm soil depth interval, bulk density was not significantly different across study sites (Table 3). However, in the 60–100 cm soil layer, bulk density in the 1st teff-Acacia agroforestry rotation was significantly greater than in the teff fields (p = 0.013), whereas bulk density in the 3rd teff-Acacia agroforestry rotation was significantly lower than in the teff fields (p < 0.0001).
3.5 Soil organic carbon contents and stocks in teff fields and teff-Acacia agroforestry (Site II)
At 0–100 cm soil depth interval, SOC content was not significantly different between teff fields and the 1st teff-Acacia agroforestry rotation (Table 4). In contrast, SOC content in the 2nd and 3rd teff-Acacia agroforestry rotation was significantly higher than in the teff fields (all P < 0.05). At 0–10 cm soil depth, SOC content was significantly higher in the 2nd teff-Acacia rotation than in the 1st teff-Acacia rotation, whereas below this soil depth interval, SOC content was not significantly different between the 1st and 2nd teff-Acacia rotation. Across sites, SOC content significantly decreased as soil depth increased (all p < 0.05) (Table 4).
There was no significant difference in SOC stocks (0–100 cm soil depth) between teff fields and the 1st teff-Acacia agroforestry rotation (Table 4). However, SOC stocks in the 2nd rotation teff-Acacia agroforestry and 3rd rotation teff-Acacia agroforestry were 159% and 244% higher than in the teff fields, respectively (p < 0.05) (Table 4). SOC stocks were significantly higher in the 3rd teff-Acacia agroforestry rotation than in the 1st rotation (p = 0.012) (Table 4). There was a significant correlation between the duration of practicing teff-Acacia agroforestry and SOC stocks (p < 0.05), showing an apparent increase of SOC stocks of 21.1 Mg ha–1 yr–1 over a period of 12 years (Fig. 2 and 3).
Aerial photos showing conversion from teff fields to teff-Acacia agroforestry in northwestern Ethiopia (Photo source: Google Earth). A Conventional teff fields; B 1st year of teff-Acacia agroforestry; C 4th year of teff-Acacia agroforestry before tree harvesting; D 4th year of teff-Acacia agroforestry after charcoal production, black dots in the fields indicate charcoal production spots.
4 Discussion
4.1 Soil characteristics in charcoal production spots in teff-Acacia agroforestry
In this study, soil pH, black carbon, SOC, and soil P and K contents were significantly higher inside than outside charcoal production spots in teff-Acacia agroforestry. Previous studies have similarly demonstrated that charcoal production spots had significantly higher soil carbon and nutrient contents compared to outside charcoal production spots in various regions: Amazon (Coomes and Miltner 2017; Miltner and Coomes 2015), China (Niu et al. 2015), and Ethiopia (Berihun et al. 2017; Bayabil et al. 2015). In southwestern Ethiopia, charcoal production spots had higher soil pH, SOC and nutrient contents, including N, P, and K, compared to adjacent cultivated areas (Nigussie and Kissi 2011). In south Ethiopia, application of charcoal produced in traditional earth mound kilns from corncobs and Lantana camara increased soil pH, SOC, N, P and K (Berihun et al. 2017). Global meta-analyses found that biochar amendment increased SOC content by 40% (Bai et al. 2019; Liu et al. 2016) and resulted in an overall positive effect of biochar on soil P availability (Glaser and Lehr 2019).
The increased soil pH at charcoal production spots could be attributed to alkaline elements such as sodium and potassium derived from ash produced during charring (Glaser et al. 2002). In addition, charcoal is known to increase soil pH due to its alkaline nature and high pH buffering capacity (Kavitha et al. 2018; Dai et al. 2017). This is supported by the observed pH of A. decurrens-derived charcoal of 7.9 (pHH2O) and 7.6 (pHKCl).
The increased SOC content at charcoal production spots could be mainly attributed to high C content of charcoal and its prevailing polycondensed aromatic structure (Glaser et al. 1998) making it more resistant to microbial degradation compared to other SOM compounds (Wang et al. 2016; Lorenz and Lal 2014). In this study, increase of stable black carbon (164%) was much higher compared to increase of SOC (48%) in the charcoal production spots (Table 1). This demonstrates that teff-Acacia agroforestry rotation, which results in increased carbon in the charcoal production spots, is an example of achieving much more stable SOC compared to other efforts to increase SOC in the context of long-term C sequestration. On the other hand, SOM can be burned during charcoal production, resulting in the reduction of SOC and STN, since it is known that fire can affect soil properties including SOC and STN (Poirier et al. 2014; Hamman et al. 2008). However, in this study, there was no evidence showing decreases of SOC and STN during charcoal production (Table 1). This is supported by global meta-analyses of Nave et al. (2011) and Boerner et al. (2009), who found that fires did not have significant overall effects on SOC and STN. Similarly, previous studies conducted in Ethiopia found that fire did not affect SOC and STN (Terefe and Kim 2020; Kim et al. 2016b). However, it should be noted that fire is different from charcoal production with respect to temperature and oxygen supply.
The increased P and K contents at charcoal production spots could be attributed to the contribution of charcoal itself (Glaser et al. 2002). The observed P and K content of A. decurrens-derived charcoal of 38 and 380 g kg-1, respectively, supports this. Another potential explanation could be an improvement of nutrient availability in the presence of charcoal (Glaser and Lehr 2019). Charcoal has the ability to retain soil nutrients due to its large surface area, high porosity, and high quantity of functional groups (Kavitha et al. 2018; Dai et al. 2017).
4.2 Effects of teff-Acacia agroforestry practices on soil carbon and nitrogen
Practicing teff-Acacia agroforestry following conversion from conventional teff fields commonly increased SOC and STN. At site I, practicing teff-Acacia agroforestry for 8 years increased SOC and STN contents (0–10 cm) by 112–169% and 100–131%, respectively. At site II, practicing teff-Acacia agroforestry for 12 years increased SOC stocks (0–100 cm) by 244%, with an increase rate of 21.1 Mg C ha–1 yr–1. The SOC increase was higher than results from previous agroforestry studies investigating changes in SOC stocks after conversion of crop fields to agroforestry. Global meta-analyses (Muchane et al. 2020; Feliciano et al. 2018; Kim and Kirschbaum 2015) and Ethiopian observations (Negash and Starr 2015; Gelaw et al. 2014) reported 10–40% increase of SOC stocks after conversion of crop fields to agroforestry. A global meta-analysis estimated that 7.2 Mg C ha–1 yr–1 can be sequestered in 14 years following agroforestry establishment (Kim et al. 2016a). Another global meta-analysis found that crop-tree intercropping and silvopasture can lead to soil carbon sequestration of 0.8 Mg C ha−1 yr−1 and 6.5 Mg C ha−1 yr−1, respectively (Feliciano et al. 2018). SOC sequestration of agroforestry systems in SSA has been documented to amount up to 14 Mg C ha–1 yr–1 (0−100 cm; Corbeels et al. 2019).
The STN increase in teff-Acacia agroforestry demonstrated in this study was also greater than documented in previous studies. A global meta-analysis found that soil N increased by 8.7% after the conversion of crop fields to tree–crop intercropping (Kim et al. 2016a). Another global meta-analysis (Muchane et al. 2020) revealed that soil N stocks under agroforestry were 46% higher than crop monocultures in the humid and sub-humid tropics. A meta-analysis of SSA studies (Kuyah et al. 2019) found that agroforestry increased soil N by 20%.
The large increase of SOC and STN of teff-Acacia agroforestry in our study may be attributed to the interaction of various factors: 1) the presence of A. decurrens with input of aboveground (leaf litter) and belowground (root litter) plant residues; 2) soil cover with grass and teff, further increasing the input of plant residues, especially belowground; 3) reduced soil disturbance, thereby reducing SOM mineralization; and 4) input of charcoal debris.
A. decurrens may play an important role in increasing STN. A. decurrens can fix atmospheric nitrogen through symbiosis with gram-negative heterotrophic rhizobacteria, which provide plant-available nitrogen (ammonium and nitrate) to legume trees such as Acacia (Molla and Linger 2017; Brockwell et al. 2005). Previous studies found that biological nitrogen fixation of Acacia species reached up to 200 kg N ha–1 yr–1 (Brockwell et al. 2005). The global average for biological nitrogen fixation of agroforestry was 246 kg N ha–1 yr−1, and biological nitrogen fixation was the highest in improved fallow, ranging from 300 to 650 kg N ha–1 yr−1 (Nygren et al. 2012). Not only naturally fallen litter from A. decurrens trees, but also branches, litter, and roots remaining on and in the soil after harvest of A. decurrens trees may partially decompose in situ, while the remaining is incorporated into SOM being composed of SOC and STN (Lal 2004; Li et al. 2012). In northwestern Ethiopia, STN was significantly higher under A. decurrens trees than in open areas located at a distance of 10 m from the trees (Molla and Linger 2017).
Grasses growing beside A. decurrens trees (grass—A. decurrens silvopasture periods) may also contribute to increased SOC and STN. The decomposition of grass litter and roots can introduce organic matter into the soil, resulting in increased SOC and STN in grasslands (Tian et al. 2018; Schipper et al. 2017). Global meta-analyses found that soil C stocks increased by nearly 50% (Kim and Kirschbaum 2015) or 0.30 Mg C ha–1 yr–1 (Deng et al. 2016) after the conversion from croplands to grasslands. A meta-analysis of studies conducted in China reported that SOC and soil inorganic N increased by 29% and 24%, respectively, when croplands were converted to grasslands (Tian et al. 2018). The silvopastoral system has been found to increase SOC (Feliciano et al. 2018; Seddaiu et al. 2013). A global meta-analysis by Feliciano et al. (2018) found that the silvopastoral system sequestered 4.4 Mg C ha−1 yr−1. Similar to grass, teff residues and teff roots can introduce organic matter into the soil resulting in increased SOC and STN (Tormena et al. 2017; Turmel et al. 2015).
Reduced soil disturbance in teff-Acacia agroforestry may result in the reduced losses of SOC and STN relative to losses observed in conventional teff fields. In conventional teff cropping in the area, the field is ploughed four times per year to a depth of 15 to 20 cm and trampled once before sowing teff. After harvest, cattle enter the field to forage for remaining crop residues. Soil disturbance caused by ploughing and trampling may enhance rates of oxidation and decomposition of SOM, resulting in higher losses of SOC and STN compared to unploughed soil (Yimer and Abdelkadir 2011; Batey 2009). In contrast, in teff-Acacia agroforestry, soil disturbance only occurs in the 1st year, with no further soil disturbance in the following three years: grass is cut and removed and no cattle are allowed to enter the field. In addition, in the teff-Acacia agroforestry system, grasses and trees can prevent soil erosion and runoff by i) slowing runoff and capturing sediments, ii) reducing wind speeds near the surface, iii) increasing infiltration by maintaining greater soil porosity, iv) protecting the surface from raindrop impacts due to their canopy and litter layer, and v) enhancing soil structural stability through increased belowground organic inputs and increased biological activity of soil macrofauna (Muchane et al. 2020; Isaac and Borden 2019; Fonte et al. 2010). For instance, in northeast Thailand, grass barriers (Vetiveria zizanioides, Brachiaria ruziziensis) and hedgerow (Leucaena leucocephala) reduced N losses by runoff, soil loss, and leaching from 55 kg N ha–1 to 37–40 kg N ha–1 (Pansak et al. 2008). A recent meta-analysis found that agroforestry trees reduced soil erosion and runoff by 50% and 57%, respectively, compared to crop monoculture (Muchane et al. 2020).
As discussed above, charcoal debris remaining on the soil surface after charcoal production may contribute to increased stable (black carbon) and total SOC. In addition, previous studies have found that biochar application not only can reduce the potential N losses induced by runoff and leaching (Razzaghi et al. 2020; Borchard et al. 2019), ammonia volatilization (Sha et al. 2019; Taghizadeh-Toosi et al. 2012), and nitrous oxide (N2O) emission (Borchard et al. 2019; Cayuela et al. 2014) but also can enhance available N inputs derived from symbiotic and nonsymbiotic biological nitrogen fixation (Ahmad et al. 2021; Rondon et al. 2007). Around 8 kg of charcoal debris remains in the charcoal production spot (diameter of around 3 m), which is equivalent to a charcoal application rate of 11 Mg ha–1 in the spot. Although the application amount is comparable to studies showing significant increase of SOC, BNF, and STN following biochar application (Ahmad et al. 2021; Wang et al. 2020), the charcoal production spots cover only 1 to 2% of the fields and the charcoal production occurs every 4 years (equivalent to a charcoal application rate of 0.01–0.02 Mg ha–1 every 4 years). Therefore, the contribution of charcoal debris to the increase SOC and STN is by far lower than the contribution of A. decurrens trees and grasses at least in the 12-year time frame studied here.
4.3 Agroforestry practice implications for carbon sequestration and livelihood
The observed increase of SOC and nutrients in teff-Acacia agroforestry has important implications for enhancing carbon sequestration and improving livelihoods in smallholder farming in developing countries.
Locally adopted agroforestry exhibits great potential for improving soil fertility and carbon sequestration. One of the barriers for smallholder farmers to improve soil fertility and carbon sequestration may be the lack of relevant knowledge and experience in applying appropriate soil management (Kim et al. 2021; Brown et al. 2018). Technology transfer also remains a challenge for smallholder farmers in developing countries since there is generally little institutional capacity and infrastructure supporting extension programs (Zerssa et al. 2021; Brown et al. 2018). Although various advantages and benefits of agroforestry including enhanced soil fertility and carbon sequestration have been identified (Muchane et al. 2020; Kim and Kirschbaum 2015), there are many barriers to implementing agroforestry under real-world conditions (Mbow et al. 2014; Rioux 2012). The current study, however, shows that locally adopted agroforestry practices can cope with these issues and increase soil C and nutrients, contributing to improved soil fertility and carbon sequestration.
Biochar can be produced using locally available feedstocks and techniques, contributing to enhanced soil carbon and fertility. Biochar has great potential for enhancing soil fertility, soil carbon sequestration, and agricultural production, and mitigating GHG emissions (Jeffery et al. 2016; Glaser and Birk 2012). However, producing biochar can be a challenge if biomass for biochar production (e.g., crop residues, biomass, garden wastes) competes with alternative utilization forms, such as construction materials, animal feed, and nutrient sources for agriculture (Gwenzi et al. 2015; Leach et al. 2012). In addition, biochar production usually requires advanced equipment and energy supply, which are generally lacking in developing countries (Gwenzi et al. 2015; Leach et al. 2012). In these respects, teff-Acacia agroforestry provides a good example of producing charcoal for energy purposes utilizing locally available biomass and techniques while simultaneously utilizing charcoal debris as biochar to enhance soil carbon and fertility.
Diversifying agricultural practices with multiple purposes and functions rather than mono-purpose practices can be a good approach to improved soil fertility and livelihood (Pretty et al. 2018; Waha et al. 2018). While conventional cropping produces a specific crop continuously, teff-Acacia agroforestry can produce crop, grass, and charcoal, which are sources of food, animal feed, energy, and cash income, thereby contributing to improved livelihood.
4.4 Limitation and suggestions for further studies
In this study, a space-for-time substitution approach was applied to analyze changes in SOC and soil nutrient contents following conversion of a conventional teff monoculture to teff-Acacia agroforestry. To reduce potential uncertainties caused by this approach, the study was carefully designed (e.g., adjacently located multiple sites, large number of replications, same soil type, microclimate, and land-use history). However, the fundamental limitation of the space-for-time substitution approach cannot be ignored, as there is always the potential bias of spatial heterogeneity in the comparison between adjacent or neighboring fields (Damgaard 2019). Therefore, to accurately quantify changes in SOC and soil nutrient contents following the conversion, it is suggested to apply a real chronosequence approach (i.e., to continuously monitor changes in SOC and soil nutrient contents after the conversion in the long term) in further studies.
In this paper, we discussed potential sources and mechanisms of increasing soil carbon and nutrients. Further efforts are needed to identify or quantify major sources of SOM and nutrients as well as mechanisms and control factors.
This study found that charcoal debris remaining in the soil increased soil pH, P, and K contents, but the effects were only found to be significant at charcoal production spots. The results suggest that additional charcoal application beyond charcoal production spots can enhance the benefits. Previous studies found that mixing biochar with compost, manure, and urine can improve soil fertility and soil carbon sequestration (Xiao et al. 2017; Agegnehu et al. 2017; Glaser et al. 2015). Therefore, it is worthwhile assessing the effect of mixing charcoal debris with other nutrient sources such as compost, manure, or urine in agroforestry practices.
As a result of conversion of teff monocropping to teff-Acacia agroforestry, synthetic fertilizer (e.g., DAP) application is reduced since synthetic fertilizer is not applied in agroforestry practices. Consequently, N2O emission might also be reduced. However, previous studies found that N2O emission increased in agroforestry with N-fixing trees due to their greater N supply to the soil (Hall et al. 2006; Chikowo et al. 2004). The results suggest that agroforestry practices with A. decurrens may increase N2O emissions. However, biochar addition is known to significantly reduce N2O emissions (Liu et al. 2019; He et al. 2017). Therefore, GHG emissions during the charcoal production process should be accurately determined, and the result should be accounted for determining the effect of conversion of teff monocropping to the agroforestry practices on GHG emissions.
5 Conclusions
We investigated locally adopted teff-Acacia agroforestry practices with sequential A. decurrens-based intercropping, silvopasture, and A. decurrens plantation with subsequent on-site charcoal production in northwestern Ethiopia. The results clearly indicate that charcoal debris remaining in the fields after charcoal production increased soil pH, black carbon, SOC, and soil P and K contents, and that teff-Acacia agroforestry practices largely increased SOC and STN. The results suggest that locally adopted teff-Acacia agroforestry practices increase total and stable SOC and soil nutrients. This study clearly demonstrates that locally adopted agroforestry practices can contribute to enhancing soil fertility and improving climate change mitigation strategies via carbon sequestration. Further studies are required i) to identify major mechanisms and control factors for the increase of soil carbon and nutrients, ii) to assess the effect of conversion from mono-cropping to agroforestry practices on GHG emissions, and iii) to improve efficiency of charcoal application.
Data availability
All data analyzed in the present study are available from the corresponding author upon reasonable request.
Code availability
Not applicable
References
Abeje MT, Tsunekawa A, Adgo E, Haregeweyn N, Nigussie Z, Ayalew Z, Elias A, Molla D, Berihun D (2019) Exploring drivers of livelihood diversification and its effect on adoption of sustainable land management practices in the Upper Blue Nile Basin, Ethiopia. Sustainability 11:2991. https://doi.org/10.3390/su11102991
Agegnehu G, Srivastava AK, Bird MI (2017) The role of biochar and biochar-compost in improving soil quality and crop performance: a review. Appl Soil Ecol 119:156–170. https://doi.org/10.1016/j.apsoil.2017.06.008
Ahmad Z, Mosa A, Zhan L, Gao B (2021) Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: a critical review. Chemosphere 278:130378. https://doi.org/10.1016/j.chemosphere.2021.130378
Akinnifesi FK, Ajayi OC, Sileshi G, Chirwa PW, Chianu J (2010) Fertiliser trees for sustainable food security in the maize-based production systems of East and Southern Africa. A review. Agron Sustain Develop 30:615–629. https://doi.org/10.1051/agro/2009058
Bai X, Huang Y, Ren W, Coyne M, Jacinthe P-A, Tao B, Hui D, Yang J, Matocha C (2019) Responses of soil carbon sequestration to climate-smart agriculture practices: a meta-analysis. Global Change Biol 25:2591–2606. https://doi.org/10.1111/gcb.14658
Batey T (2009) Soil compaction and soil management- a review. Soil Use Manag 25:335–345. https://doi.org/10.1111/j.1475-2743.2009.00236.x
Bayabil HK, Stoof CR, Lehmann JC, Yitaferu B, Steenhuis TS (2015) Assessing the potential of biochar and charcoal to improve soil hydraulic properties in the humid Ethiopian Highlands: The Anjeni watershed. Geoderma 243-244:115–123. https://doi.org/10.1016/j.geoderma.2014.12.015
Belayneh Y, Ru G, Guadie A, Teffera ZL, Tsega M (2020) Forest cover change and its driving forces in Fagita Lekoma District, Ethiopia. J For Res 31:1567–1582. https://doi.org/10.1007/s11676-018-0838-8
Berihun T, Tolosa S, Tadele M, Kebede F (2017) Effect of biochar application on growth of Garden Pea (Pisum sativum L.) in acidic soils of Bule Woreda Gedeo Zone Southern Ethiopia. Int J Agron 2017:6827323–6827328. https://doi.org/10.1155/2017/6827323
Berihun ML, Tsunekawa A, Haregeweyn N, Meshesha DT, Adgo E, Tsubo M, Masunaga T, Fenta AA, Sultan D, Yibeltal M (2019) Exploring land use/land cover changes, drivers and their implications in contrasting agro-ecological environments of Ethiopia. Land Use Policy 87:104052. https://doi.org/10.1016/j.landusepol.2019.104052
Birhane E, Teklay R, Gebrehiwet K, Solomon N, Tadesse T (2019) Maintaining Acacia polyacantha trees in farmlands enhances soil fertility and income of farmers in North Western Tigray, Northern Ethiopia. Agrofor Syst 93:2135–2149. https://doi.org/10.1007/s10457-018-0328-1
Bockarie AS, Marais EA, MacKenzie AR (2020) Air pollution and climate forcing of the charcoal industry in Africa. Environ Sci Technol 54:13429–13438. https://doi.org/10.1021/acs.est.0c03754
Boerner RE, Huang J, Hart SC (2009) Impacts of fire and fire surrogate treatments on forest soil properties: a meta-analytical approach. Ecol Appl 19:338–358. https://doi.org/10.1890/07-1767.1
Borchard N, Schirrmann M, Cayuela ML, Kammann C, Wrage-Mönnig N, Estavillo JM, Fuertes-Mendizábal T, Sigua G, Spokas K, Ippolito JA, Novak J (2019) Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: a meta-analysis. Sci Tot Environ 651:2354–2364. https://doi.org/10.1016/j.scitotenv.2018.10.060
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analyses of soils. Agron J 54:464–465. https://doi.org/10.2134/agronj1962.00021962005400050028x
Brockwell J, Searle SD, Jeavons AC, Waayers M (2005) Nitrogen fixation in acacias: an untapped resource for sustainable plantations, farm forestry and land reclamation. ACIAR Monograph No. 115: 132p
Brown B, Nuberg I, Llewellyn R (2018) Constraints to the utilisation of conservation agriculture in Africa as perceived by agricultural extension service providers. Land Use Policy 73:331–340. https://doi.org/10.1016/j.landusepol.2018.02.009
Cayuela M, Van Zwieten L, Singh B, Jeffery S, Roig A, Sánchez-Monedero M (2014) Biochar's role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16. https://doi.org/10.1016/j.agee.2013.10.009
Chidumayo EN, Gumbo DJ (2013) The environmental impacts of charcoal production in tropical ecosystems of the world: a synthesis. Energy Sustain Dev 17:86–94. https://doi.org/10.1016/j.esd.2012.07.004
Chikowo R, Mapfumo P, Nyamugafata P, Giller KE (2004) Mineral N dynamics, leaching and nitrous oxide losses under maize following two-year improved fallows on a sandy loam soil in Zimbabwe. Plant Soil 259:315–330. https://doi.org/10.1023/B:PLSO.0000020977.28048.fd
Coomes OT, Miltner BC (2017) Indigenous charcoal and biochar production: potential for soil improvement under shifting cultivation systems. Land Degrad Dev 28:811–821. https://doi.org/10.1002/ldr.2500
Corbeels M, Cardinael R, Naudin K, Guibert H, Torquebiau E (2019) The 4 per 1000 goal and soil carbon storage under agroforestry and conservation agriculture systems in sub-Saharan Africa. Soil Till Res 188:16–26. https://doi.org/10.1016/j.still.2018.02.015
Dai Z, Zhang X, Tang C, Muhammad N, Wu J, Brookes PC, Xu J (2017) Potential role of biochars in decreasing soil acidification - a critical review. Sci Tot Environ 581-582:601–611. https://doi.org/10.1016/j.scitotenv.2016.12.169
Damgaard C (2019) A critique of the space-for-time substitution practice in community ecology. Trends Ecol Evol 34:416–421. https://doi.org/10.1016/j.tree.2019.01.013
Deng L, Zhu GY, Tang ZS, Shangguan ZP (2016) Global patterns of the effects of land-use changes on soil carbon stocks. Glob Ecol.Conserv 5:127–138. https://doi.org/10.1016/j.gecco.2015.12.004
Elias E (2016) Soils of the Ethiopian highlands: geomorphology and properties. Wageningen University and Research Centre (Wageningen UR), The Netherlands, 385pp
Feliciano D, Ledo A, Hillier J, Nayak DR (2018) Which agroforestry options give the greatest soil and above ground carbon benefits in different world regions? Agric Ecosys Environ 254:117–129. https://doi.org/10.1016/j.agee.2017.11.032
Fonte SJ, Barrios E, Six J (2010) Earthworms, soil fertility and aggregate-associated soil organic matter dynamics in the Quesungual agroforestry system. Geoderma 155:320–328. https://doi.org/10.1016/j.geoderma.2009.12.016
Gelaw AM, Singh BR, Lal R (2014) Soil organic carbon and total nitrogen stocks under different land uses in a semiarid watershed in Tigray, Northern Ethiopia. Agric Ecosyst Environ 188:256–263. https://doi.org/10.1016/j.agee.2014.02.035
Glaser B, Birk JJ (2012) State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de índio). Geochim Cosmochim Acta 82:39–51. https://doi.org/10.1016/j.gca.2010.11.029
Glaser B, Lehr VI (2019) Biochar effects on phosphorus availability in agricultural soils: a meta-analysis. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-45693-z
Glaser B, Haumaier L, Guggenberger G, Zech W (1998) Black carbon in soils: the use of benzenecarboxylic acids as specific markers. Org Geochem 29:811–819. https://doi.org/10.1016/S0146-6380(98)00194-6
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal - a review. Biol Fertil Soils 35:219–230. https://doi.org/10.1007/s00374-002-0466-4
Glaser B, Wiedner K, Seelig S, Schmidt HP, Gerber H (2015) Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron Sustain Develop 35:667–678. https://doi.org/10.1007/s13593-014-0251-4
Grossman RB, Reinsch TG (2002) Bulk density and linear extensibility. In: Dane JH, Topp GC (eds) Methods of soil analysis. Part 4 physical methods. Soil Science Society of America, Inc. and American Society of Agronomy, Inc., Madison, pp 201–254
Gwenzi W, Chaukura N, Mukome FN, Machado S, Nyamasoka B (2015) Biochar production and applications in sub-Saharan Africa: opportunities, constraints, risks and uncertainties. J Environ Manag 150:250–261. https://doi.org/10.1016/j.jenvman.2014.11.027
Hall NM, Kaya B, Dick J, Skiba U, Niang A, Tabo R (2006) Effect of improved fallow on crop productivity, soil fertility and climate-forcing gas emissions in semi-arid conditions. Biol Fertil Soils 42:224–230. https://doi.org/10.1007/s00374-005-0019-8
Hamman ST, Burke IC, Knapp EE (2008) Soil nutrients and microbial activity after early and late season prescribed burns in a Sierra Nevada mixed conifer forest. For Ecol Manag 256:367–374. https://doi.org/10.1016/j.foreco.2008.04.030
He Y, Zhou X, Jiang L, Li M, Du Z, Zhou G, Shao J, Wang X, Xu Z, Hosseini Bai S (2017) Effects of biochar application on soil greenhouse gas fluxes: a meta-analysis. GCB Bioenergy 9:743–755. https://doi.org/10.1111/gcbb.12376
Intergovernmental Panel on Climate Change (IPCC) (2003) Good practice guidance for land use, land use change and forestry. In: Penman J, Gytarsky M, Hiraishi T, Krug T, Kruger D, Pipatti R, Buendia L, Miwa K, Ngara T, Tanabe K, Wagner F (eds) IPCC/OECD/IEA/IGES. Hayama, Japan
Isaac ME, Borden KA (2019) Nutrient acquisition strategies in agroforestry systems. Plant Soil 444:1–19. https://doi.org/10.1007/s11104-019-04232-5
Jeffery S, Verheijen FGA, Kammann C, Abalos D (2016) Biochar effects on methane emissions from soils: a meta-analysis. Soil Biol Biochem 101:251–258. https://doi.org/10.1016/j.soilbio.2016.07.021
Jose S, Gold MA, Garrett HE (2012) The future of temperate agroforestry in the United States. In: Nair PKR, Garrity D (eds) Agroforestry - the future of global land use. Springer Netherlands, Dordrecht, pp 217–245. https://doi.org/10.1007/978-94-007-4676-3_14
Kavitha B, Reddy PVL, Kim B, Lee SS, Pandey SK, Kim K-H (2018) Benefits and limitations of biochar amendment in agricultural soils: a review. J Environ Manag 227:146–154. https://doi.org/10.1016/j.jenvman.2018.08.082
Kim D-G, Kirschbaum MU (2015) The effect of land-use change on the net exchange rates of greenhouse gases: a compilation of estimates. Agric Ecosyst Environ 208:114–126. https://doi.org/10.1016/j.agee.2015.04.026
Kim D-G, Kirschbaum MUF, Beedy TL (2016a) Carbon sequestration and net emissions of CH4 and N2O under agroforestry: Synthesizing available data and suggestions for future studies. Agric Ecosyst Environ 226:65–78. https://doi.org/10.1016/j.agee.2016.04.011
Kim D-G, Taddese H, Belay A, Kolka R (2016b) The impact of traditional fire management on soil carbon and nitrogen pools in a montane forest, southern Ethiopia. Int J Wildland Fire 25:1110–1116. https://doi.org/10.1071/WF16022
Kim D-G, Grieco E, Bombelli A, Hickman JE, Sanz-Cobena A (2021) Challenges and opportunities for enhancing food security and greenhouse gas mitigation in smallholder farming in sub-Saharan Africa. A review. Food Secur 13:457–476. https://doi.org/10.1007/s12571-021-01149-9
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am StatAss 47:583–621 https://www.jstor.org/stable/2280779
Kuyah S, Whitney CW, Jonsson M, Sileshi GW, Öborn I, Muthuri CW, Luedeling E (2019) Agroforestry delivers a win-win solution for ecosystem services in sub-Saharan Africa. A meta-analysis. Agron Sustain Dev 39:47. https://doi.org/10.1007/s13593-019-0589-8
Lal R (2004) Soil carbon sequestration to mitigate climate change. Geoderma 123:1–22. https://doi.org/10.1016/j.geoderma.2004.01.032
Leach M, Fairhead J, Fraser J (2012) Green grabs and biochar: revaluing African soils and farming in the new carbon economy. J Peasant Stud 39:285–307. https://doi.org/10.1080/03066150.2012.658042
Li DJ, Niu SL, Luo YQ (2012) Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: a meta-analysis. New Phytol 195:172–181. https://doi.org/10.1111/j.1469-8137.2012.04150.x
Liu S, Zhang Y, Zong Y, Hu Z, Wu S, Zhou J, Jin Y, Zou J (2016) Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: a meta-analysis. GCB Bioenergy 8:392–406. https://doi.org/10.1111/gcbb.12265
Liu Q, Liu B, Zhang Y, Hu T, Lin Z, Liu G, Wang X, Ma J, Wang H, Jin H, Ambus P, Amonette JE, Xie Z (2019) Biochar application as a tool to decrease soil nitrogen losses (NH3 volatilization, N2O emissions, and N leaching) from croplands: options and mitigation strength in a global perspective. Glob Change Biol 25:2077–2093. https://doi.org/10.1111/gcb.14613
Lorenz K, Lal R (2014) Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J Plant Nutr Soil Sci 177:651–670. https://doi.org/10.1002/jpln.201400058
Majumder S, Neogi S, Dutta T, Powel MA, Banik P (2019) The impact of biochar on soil carbon sequestration: meta-analytical approach to evaluating environmental and economic advantages. J Environ Manag 250:109466. https://doi.org/10.1016/j.jenvman.2019.109466
Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Ann Stat 18:50–60 https://www.jstor.org/stable/2236101
Mbow C, Smith P, Skole D, Duguma L, Bustamante M (2014) Achieving mitigation and adaptation to climate change through sustainable agroforestry practices in Africa. Curr Opin Environ Sustain 6:8–14. https://doi.org/10.1016/j.cosust.2013.09.002
Miltner BC, Coomes OT (2015) Indigenous innovation incorporates biochar into swidden-fallow agroforestry systems in Amazonian Peru. Agrofor Syst 89:409–420. https://doi.org/10.1007/s10457-014-9775-5
Molla A, Linger E (2017) Effects of Acacia decurrens (Green wattle) tree on selected soil physico-chemical properties North-western Ethiopia. Res J Agric Environ Manag 6:095–103 http://www.apexjournal.org/rjaem/archive/2017/July/fulltext/Molla%20and%20Linger.pdf
Motulsky HJ, Christopoulos A (2004) Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford University Press, New York
Muchane MN, Sileshi GW, Gripenberg S, Jonsson M, Pumarino L, Barrios E (2020) Agroforestry boosts soil health in the humid and sub-humid tropics: a meta-analysis. Agric Ecosyst Environ 295:106899. https://doi.org/10.1016/j.agee.2020.106899
Nave LE, Vance ED, Swanston CW, Curtis PS (2011) Fire effects on temperate forest soil C and N storage. Ecol Appl 21:1189–1201. https://doi.org/10.1890/10-0660.1
Negash M, Starr M (2015) Biomass and soil carbon stocks of indigenous agroforestry systems on the south-eastern Rift Valley escarpment, Ethiopia. Plant Soil 393:95–107. https://doi.org/10.1007/s11104-015-2469-6
Nigussie A, Kissi E (2011) Impact of biomass burning on physicochemical properties of Nitisol in the Southwestern Ethiopia. Asian J Agric Res 5:223–233 https://doi.org/10.3923/ajar.2011.223.233
Nigussie Z, Tsunekawa A, Haregeweyn N, Adgo E, Nohmi M, Tsubo M, Aklog D, Meshesha DT, Abele S (2017) Factors affecting small-scale farmers' land allocation and tree density decisions in an acacia decurrens-based taungya system in Fagita Lekoma District, North-Western Ethiopia. Small-scale For 16:219–233. https://doi.org/10.1007/s11842-016-9352-z
Nigussie Z, Tsunekawa A, Haregeweyn N, Adgo E, Tsubo M, Ayalew Z, Abele S (2020) Economic and financial sustainability of an Acacia decurrens-based Taungya system for farmers in the Upper Blue Nile Basin, Ethiopia. Land Use Policy 90:104331. https://doi.org/10.1016/j.landusepol.2019.104331
Nigussie Z, Tsunekawa A, Haregeweyn N, Tsubo M, Adgo E, Ayalew Z, Abele S (2021) The impacts of Acacia decurrens plantations on livelihoods in rural Ethiopia. Land Use Policy 100:104928. https://doi.org/10.1016/j.landusepol.2020.104928
Niu L-Q, Jia P, Li S-P, Kuang J-L, He X-X, Zhou W-H, Liao B, Shu W-S, Li J-T (2015) Slash-and-char: an ancient agricultural technique holds new promise for management of soils contaminated by Cd, Pb and Zn. Environ Pollut 205:333–339. https://doi.org/10.1016/j.envpol.2015.06.017
Nygren P, Fernández MP, Harmand J-M, Leblanc HA (2012) Symbiotic dinitrogen fixation by trees: an underestimated resource in agroforestry systems? Nutr Cycl Agroecosyst 94:123–160. https://doi.org/10.1007/s10705-012-9542-9
Pansak W, Hilger TH, Dercon G, Kongkaew T, Cadisch G (2008) Changes in the relationship between soil erosion and N loss pathways after establishing soil conservation systems in uplands of Northeast Thailand. Agric Ecosyst Environ 128:167–176. https://doi.org/10.1016/j.agee.2008.06.002
Poirier V, Paré D, Boiffin J, Munson AD (2014) Combined influence of fire and salvage logging on carbon and nitrogen storage in boreal forest soil profiles. For Ecol Manag 326:133–141. https://doi.org/10.1016/j.foreco.2014.04.021
Pretty J, Benton TG, Bharucha ZP, Dicks LV, Flora CB, Godfray HCJ, Goulson D, Hartley S, Lampkin N, Morris C, Pierzynski G, Prasad PVV, Reganold J, Rockström J, Smith P, Thorne P, Wratten S (2018) Global assessment of agricultural system redesign for sustainable intensification. Nat Sustain 1:441–446. https://doi.org/10.1038/s41893-018-0114-0
Razzaghi F, Obour PB, Arthur E (2020) Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 361:114055. https://doi.org/10.1016/j.geoderma.2019.114055
Rioux J (2012) Opportunities and challenges of promoting agroforestry for climate change mitigation: a case-study of the Mitigation of Climate Change in Agriculture (MICCA) pilot project in Tanzania. Nature Faune 26:63–68 http://www.fao.org/climatechange/36653-0d7b3e802032f0279b368b3536cf5c3ee.pdf
Rondon MA, Lehmann J, Ramírez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soils 43:699–708. https://doi.org/10.1007/s00374-006-0152-z
Schipper LA, Mudge PL, Kirschbaum MUF, Hedley CB, Golubiewski NE, Smaill SJ, Kelliher FM (2017) A review of soil carbon change in New Zealand's grazed grasslands. NZ J Agric Res 60:93–118. https://doi.org/10.1080/00288233.2017.1284134
Schmidt H-P, Kammann C, Hagemann N, Leifeld J, Bucheli TD, Sánchez Monedero MA, Cayuela ML (2021) Biochar in agriculture – a systematic review of 26 global meta-analyses. GCB Bioenergy 13:1708–1730. https://doi.org/10.1111/gcbb.12889
Seddaiu G, Porcu G, Ledda L, Roggero PP, Agnelli A, Corti G (2013) Soil organic matter content and composition as influenced by soil management in a semi-arid Mediterranean agro-silvo-pastoral system. Agric Ecosyst Environ 167:1–11. https://doi.org/10.1016/j.agee.2013.01.002
Sha Z, Li Q, Lv T, Misselbrook T, Liu X (2019) Response of ammonia volatilization to biochar addition: A meta-analysis. Sci Total Environ 655:1387–1396. https://doi.org/10.1016/j.scitotenv.2018.11.316
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika:591–611 https://www.jstor.org/stable/2333709
Sileshi G, Akinnifesi FK, Ajayi OC, Place F (2008) Meta-analysis of maize yield response to planted fallow and green manure legumes in sub-Saharan Africa. Plant Soil 307:1–19. https://doi.org/10.1007/s11104-008-9547-y
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) A wood based low-temperature biochar captures NH3-N generated from ruminant urine-N, retaining its bioavailability. Plant Soil 353:73–84. https://doi.org/10.1007/s11104-011-1010-9
Terefe B, Kim D-G (2020) Shifting cultivation maintains but its conversion to mono-cropping decreases soil carbon and nitrogen stocks compared to natural forest in Western Ethiopia. Plant Soil 453:105–117. https://doi.org/10.1007/s11104-019-03942-0
Tian D, Xiang Y, Wang B, Li M, Liu Y, Wang J, Li Z, Niu S (2018) Cropland abandonment enhances soil inorganic nitrogen retention and carbon stock in China: A meta-analysis. Land Degrad Dev 29:3898–3906. https://doi.org/10.1002/ldr.3137
Tormena CA, Karlen DL, Logsdon S, Cherubin MR (2017) Corn stover harvest and tillage impacts on near-surface soil physical quality. Soil Till Res 166:122–130. https://doi.org/10.1016/j.still.2016.09.015
Turmel M-S, Speratti A, Baudron F, Verhulst N, Govaerts B (2015) Crop residue management and soil health: a systems analysis. Agric Syst 134:6–16. https://doi.org/10.1016/j.agsy.2014.05.009
Waha K, van Wijk MT, Fritz S, See L, Thornton PK, Wichern J, Herrero M (2018) Agricultural diversification as an important strategy for achieving food security in Africa. Glob Change Biol 24:3390–3400. https://doi.org/10.1111/gcb.14158
Wang J, Xiong Z, Kuzyakov Y (2016) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8:512–523. https://doi.org/10.1111/gcbb.12266
Wang D, Jiang P, Zhang H, Yuan W (2020) Biochar production and applications in agro and forestry systems: a review. Sci Total Environ 723:137775. https://doi.org/10.1016/j.scitotenv.2020.137775
Weil RR, Brady NC (2017) The nature and properties of soil, 15th edn. Pearson, London
Wondie M, Mekuria W (2018) Planting of Acacia decurrens and dynamics of land cover change in Fagita Lekoma District in the northwestern highlands of Ethiopia. Mt Res Dev 38:230–240. https://doi.org/10.1659/MRD-JOURNAL-D-16-00082.1
Xiao R, Awasthi MK, Li R, Park J, Pensky SM, Wang Q, Wang JJ, Zhang Z (2017) Recent developments in biochar utilization as an additive in organic solid waste composting: a review. Bioresour Technol 246:203–213. https://doi.org/10.1016/j.biortech.2017.07.090
Yimer F, Abdelkadir A (2011) Soil property changes following conversion of acacia woodland into grazing and farmlands in the Rift Valley area of Ethiopia. Land Degrad Dev 22:425–431. https://doi.org/10.1002/ldr.1022
Zerssa G, Feyssa D, Kim D-G, Eichler-Löbermann B (2021) Challenges of smallholder farming in Ethiopia and opportunities by adopting climate-smart agriculture. Agriculture 11:192. https://doi.org/10.3390/agriculture11030192
Zhu X, Jackson RD, DeLucia EH, Tiedje JM, Liang C (2020) The soil microbial carbon pump: from conceptual insights to empirical assessments. Glob Change Biol 26:6032–6039. https://doi.org/10.1111/gcb.15319
Acknowledgements
We thank researcher Tanya K. Sayyed for editing and providing useful comments to improve the paper. This work was supported by the Korea International Cooperation Agency (KOICA) under the title of strengthening the capacity to address climate change on forestry sector in Ethiopia (NO. 2018-004), International Atomic Energy Agency (IAEA) Coordinated Research Project (CRP D15020) Developing Climate Smart Agricultural practices for mitigation of greenhouse gases, and the ClimEtSan project, funded by the German Federal Ministry of Education and Research (BMBF) under the grant no. 01DG17010 and the German Academic Exchange Service (DAAD) under the grant no. 57363162.
Funding
Korea International Cooperation Agency (KOICA) under the title of strengthening the capacity to address climate change on forestry sector in Ethiopia (NO. 2018-004), International Atomic Energy Agency (IAEA) Coordinated Research Project (CRP D15020) Developing Climate Smart Agricultural practices for mitigation of greenhouse gases, and the ClimEtSan project, funded by the German Federal Ministry of Education and Research (BMBF) under the grant no. 01DG17010 and the German Academic Exchange Service (DAAD) under the grant no. 57363162. Fire-related analysis and interpretation were supported by the German Research Foundation (DFG) under the grant no. GL 327/18-1, 2.
Author information
Authors and Affiliations
Contributions
GK, FY, D-GK, NB designed research, GK, FY, NB collected samples, GK, NB, BG analyzed samples, GK, FY, D-GK analyzed results and drafted, D-GK, GK, FY, NB, BG revised.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 21 kb)
About this article
Cite this article
Kim, DG., Kassahun, G., Yimer, F. et al. Agroforestry practices and on-site charcoal production enhance soil fertility and climate change mitigation in northwestern Ethiopia. Agron. Sustain. Dev. 42, 80 (2022). https://doi.org/10.1007/s13593-022-00810-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s13593-022-00810-7