Abstract
The present work reviews the liquid antisolvent crystallization (LASC) to prepare the nanoparticle of pharmaceutical compounds to enhance their solubility, dissolution rate, and bioavailability. The application of ultrasound and additives is discussed to prepare the particles with narrow size distribution. The use of ionic liquid as an alternative to conventional organic solvent is presented. Herbal compounds, also known for low aqueous solubility and limited clinical application, have been crystalized by LASC and discussed here. The particle characteristics such as particle size and particle size distribution are interpreted in terms of supersaturation, nucleation, and growth phenomena. To overcome the disadvantage of batch crystallization, the scientific literature on continuous flow reactors is also reviewed. LASC in a microfluidic device is emerging as a promising technique. The different design of the microfluidic device and their application in LASC are discussed. The combination of the LASC technique with traditional techniques such as high-pressure homogenization and spray drying is presented. A comparison of product characteristics prepared by LASC and the supercritical CO2 antisolvent method is discussed to show that LASC is an attractive and inexpensive alternative for nanoparticle preparation. One of the major strengths of this paper is a discussion on less-explored applications of LASC in pharmaceutical research to attract the attention of future researchers.
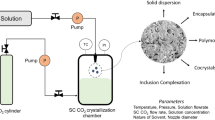
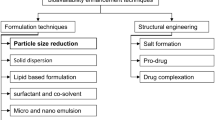
Graphical abstract








Similar content being viewed by others
Data availability
Not applicable.
References
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;413–20.
Liu Q, Mai Y, Gu X, Zhao Y, Di X, Ma X, et al. A wet-milling method for the preparation of cilnidipine nanosuspension with enhanced dissolution and oral bioavailability. J Drug Deliv Sci Technol. 2020;55:101371.
Velický M, Tam KY, Dryfe RAW. In situ artificial membrane permeation assay under hydrodynamic control: correlation between drug in vitro permeability and fraction absorbed in humans. Eur J Pharm Sci. 2011;44:299–309.
Shekhawat P, Bagul M, Edwankar D, Pokharkar V. Enhanced dissolution/caco-2 permeability, pharmacokinetic and pharmacodynamic performance of re-dispersible eprosartan mesylate nanopowder. Eur J Pharm Sci. 2019;132:72–85.
Song S, Wang C, Wang S, Siegel RA, Sun CC. Efficient development of sorafenib tablets with improved oral bioavailability enabled by coprecipitated amorphous solid dispersion. Int J Pharm. 2021;610:121216.
Lentz KA, Plum J, Steffansen B, Arvidsson PO, Omkvist DH, Pedersen AJ, et al. Predicting in vivo performance of fenofibrate amorphous solid dispersions using in vitro non-sink dissolution and dissolution permeation setup. Int J Pharm. 2021;610:121174.
Müller M, Wiedey R, Hoheisel W, Serno P, Breitkreutz J. Impact of co-administered stabilizers on the biopharmaceutical performance of regorafenib amorphous solid dispersions. Eur J Pharm Biopharm. 2021;169:189–99.
Ijaz QA, Latif S, Shoaib Q, Rashid M, Arshad MS, Hussain A, et al. Preparation and characterization of ph-independent sustained-release tablets containing hot melt extruded solid dispersions of clarithromycin: tablets containing solid dispersions of clarithromycin. AAPS PharmSciTech. 2021;22:1–12.
Koli AR, Ranch KM, Patel HP, Parikh RK, Shah DO, Maulvi FA. Oral bioavailability improvement of felodipine using tailored microemulsion: surface science, ex vivo and in vivo studies. Int J Pharm. 2021;596:120202.
Mena-Hernández J, Jung-Cook H, Llaguno-Munive M, García-López P, Ganem-Rondero A, López-Ramírez S, et al. Preparation and evaluation of mebendazole microemulsion for intranasal delivery: an alternative approach for glioblastoma treatment. AAPS PharmSciTech. 2020;21:1–12.
Shah N, Seth A, Balaraman R, Sailor G, Javia A, Gohil D. Oral bioavailability enhancement of raloxifene by developing microemulsion using D-optimal mixture design: optimization and in-vivo pharmacokinetic study. Drug Dev Ind Pharm. 2018;44:687–96.
Seok SH, Lee SA, Park ES. Formulation of a microemulsion-based hydrogel containing celecoxib. J Drug Deliv Sci Technol. 2018;43:409–14.
Shinde UA, Modani SH, Singh KH. Design and development of repaglinide microemulsion gel for transdermal delivery. AAPS PharmSciTech. 2018;19:315–25.
Pinto LMA, Adeoye O, Thomasi SS, Francisco AP, Carvalheiro MC, Cabral-Marques H. Preparation and characterization of a synthetic curcumin analog inclusion complex and preliminary evaluation of in vitro antileishmanial activity. Int J Pharm. 2020;589:119764.
Špehar TK, Pocrnić M, Klarić D, Bertoša B, Čikoš A, Jug M, et al. Investigation of praziquantel/cyclodextrin inclusion complexation by NMR and LC-HRMS/MS: mechanism, solubility, chemical stability, and degradation products. Mol Pharm. 2021;18:4210–23.
Sherje AP, Kulkarni V, Murahari M, Nayak UY, Bhat P, Suvarna V, et al. Inclusion complexation of etodolac with hydroxypropyl-beta-cyclodextrin and auxiliary agents: Formulation characterization and molecular modeling studies. Mol Pharm. 2017;14:1231–42.
Li Y, He ZD, Zheng QE, Hu C, Lai WF. Hydroxypropyl-β-cyclodextrin for delivery of baicalin via inclusion complexation by supercritical fluid encapsulation. Molecules. 2018;23.
Araújo GP, Martins FT, Taveira SF, Cunha-Filho M, Marreto RN. Effects of formulation and manufacturing process on drug release from solid self-emulsifying drug delivery systems prepared by high shear mixing. AAPS PharmSciTech. 2021;22.
Khanfar M, Al-Nimry S, Attar S. Solid self nano-emulsifying system for the enhancement of dissolution and bioavailability of Prasugrel HCl: in vitro and in vivo studies. Pharm Dev Technol. 2021;26:1021–33.
Mahajan S, Singh D, Sharma R, Singh G, Bedi N. pH-independent dissolution and enhanced oral bioavailability of aripiprazole-loaded solid self-microemulsifying drug delivery system. AAPS PharmSciTech. 2021;22.
Assi RA, Abdulbaqi IM, Ming TS, Yee CS, Wahab HA, Asif SM, et al. Liquid and solid self-emulsifying drug delivery systems (Sedds) as carriers for the oral delivery of azithromycin: Optimization, in vitro characterization and stability assessment. Pharmaceutics. 2020;12:1–29.
Fu X, Xu S, Li Z, Chen K, Fan H, Wang Y, et al. Enhanced intramuscular bioavailability of cannabidiol using nanocrystals: formulation, in vitro appraisal, and pharmacokinetics. AAPS PharmSciTech. 2022;23:1–12.
Sodeifian G, Sajadian SA, Derakhsheshpour R. CO2 utilization as a supercritical solvent and supercritical antisolvent in production of sertraline hydrochloride nanoparticles. J CO2 Util. 2022;55:101799.
Zhu Y, Fu Y, Zhang A, Wang X, Zhao Z, Zhang Y, et al. Rod-shaped nintedanib nanocrystals improved oral bioavailability through multiple intestinal absorption pathways. Eur J Pharm Sci. 2022;168.
El Sayeh F, Abou El Ela A, Abbas Ibrahim M, Alqahtani Y, Almomen A, Sfouq Aleanizy F. Fluconazole nanoparticles prepared by antisolvent precipitation technique: physicochemical, in vitro, ex vivo and in vivo ocular evaluation. Saudi Pharm J. 2021;29:576–85.
Jiang T, Han N, Zhao B, Xie Y, Wang S. Enhanced dissolution rate and oral bioavailability of simvastatin nanocrystal prepared by sonoprecipitation. Drug Dev Ind Pharm. 2012;38:1230–9.
Kumar R, Thakur AK, Chaudhari P, Banerjee N. Particle size reduction techniques of pharmaceutical compounds for the enhancement of their dissolution rate and bioavailability. J Pharm Innov. 2021;1–20.
Rabinow BE. Nanosuspensions in drug delivery. Natur Rev Drug Discov. 2004;9:785–96.
Kesisoglou F, Mitra A. Crystalline nanosuspensions as potential toxicology and clinical oral formulations for BCS II/IV Compounds. AAPS J. 2012;14:677–87.
Müller RH, Gohla S, Keck CM. State of the art of nanocrystals - special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm. 2011;1–9.
Fontana F, Figueiredo P, Zhang P, Hirvonen JT, Liu D, Santos HA. Production of pure drug nanocrystals and nano co-crystals by confinement methods. Adv Drug Deliv Rev. 2018;131:3–21.
Kumar R, Thakur AK, Banerjee N, Chaudhari P. Investigation on crystallization phenomena with supercritical carbon dioxide (CO2) as the antisolvent. Int J Chem React Eng. 2021;19:861–71.
Kumar R, Thakur AK, Banerjee N, Chaudhari P. A critical review on the particle generation and other applications of rapid expansion of supercritical solution. Int J Pharm. 2021;121089.
Kumar R, Kumar S, Chaudhari P, Thakur AK. Liquid antisolvent recrystallization and solid dispersion of flufenamic acid with polyvinylpyrrolidone K-30. Int J Chem React Eng. 2021;19:663–71.
Sinha B, Müller RH, Möschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;126–41.
Yu G, Zhu H, Huang Y, Zhang X, Sun L, Wang Y, et al. Preparation of Daidzein microparticles through liquid antisolvent precipitation under ultrasonication. Ultrason Sonochem. 2021;79:105772.
McGinty J, Chong MWS, Manson A, Brown CJ, Nordon A, Sefcik J. Effect of process conditions on particle size and shape in continuous antisolvent crystallisation of lovastatin. Curr Comput-Aided Drug Des. 2020;10:1–17.
Ha ES, Park H, Lee SK, Jeong JS, Kim JS, Kim MS. Solubility, solvent effect, and modelling of oxcarbazepine in mono-solvents and N-methyl-2-pyrrolidone + water solvent mixtures at different temperatures and its application for the preparation of nanosuspensions. J Mol Liq. 2021;339:116792.
Thakur AK, Kumar R, Vipin Kumar VK, Kumar A, Kumar Gaurav G, Naresh GK. A critical review on thermodynamic and hydrodynamic modeling and simulation of liquid antisolvent crystallization of pharmaceutical compounds. J Mol Liq. 2022;362:119663.
Yuan N, Chen Z, Suo Z, Cheng Q, Sun Q, Li Y, et al. Solubility measurement, thermodynamic modeling, and molecular dynamic simulation of regorafenib in pure and binary solvents. J Chem Thermodyn. 2022;167.
Chen S, Liu Q, Dou H, Zhang L, Pei L, Huang R, et al. Solubility and dissolution thermodynamic properties of Mequindox in binary solvent mixtures. J Mol Liq. 2020;303:112619.
Oberoi D, Shankar U, Dagar P, Sahu S, Bandyopadhyay A. Electrochromic and bipolar memory switching properties of novel Eu(III)-polymer of multidentate Schiff’s base ligand. J Mater Sci Mater Electron. 2020;31:20345–59.
Shankar U, Sethi SK, Singh BP, Kumar A, Manik G, Bandyopadhyay A. Optically transparent and lightweight nanocomposite substrate of poly(methyl methacrylate-co-acrylonitrile)/MWCNT for optoelectronic applications: an experimental and theoretical insight. J Mater Sci. 2021;56:17040–61.
Shankar U, Gupta CR, Oberoi D, Singh BP, Kumar A, Bandyopadhyay A. A facile way to synthesize an intrinsically ultraviolet-C resistant tough semiconducting polymeric glass for organic optoelectronic device application. Carbon N Y Pergamon. 2020;168:485–98.
Li T, Zhu L, Li J, Cao Z, Sha J, Li Y, et al. Solubility, thermodynamic properties and molecular simulation of tinidazole in fourteen mono-solvents at different temperatures. J Chem Thermodyn. 2022;170.
Kumar R, Rawat DS, Thakur AK, Chaudhari P, Banerjee N. Experimental measurement and thermodynamic modeling of solubility of flufenamic acid in different pure solvents. Mater Today Proc. 2022;57:1489–93.
Pabba S, Kumari A, Ravuri MG, Thella PK, Satyavathi B, Shah K, et al. Experimental determination and modelling of the co-solvent and antisolvent behaviour of binary systems on the dissolution of pharma drug; L-aspartic acid and thermodynamic correlations. J Mol Liq. 2020;314:113657.
Rathi N, Paradkar A, Gaikar VG. Polymorphs of curcumin and its cocrystals with cinnamic acid. J Pharm Sci. 2019;108:2505–16.
Rad RT, Mortazavi SA, Vatanara A, Dadashzadeh S. Enhanced dissolution rate of tadalafil nanoparticles prepared by sonoprecipitation technique: optimization and physicochemical investigation. Iran J Pharm Res. 2017;16:1335–48.
de Azevedo JR, Fabienne E, Jean-Jacques L, Inês RM. Antisolvent crystallization of a cardiotonic drug in ionic liquids: effect of mixing on the crystal properties. J Cryst Growth. 2017;472:29–34.
Valeh-e-Sheyda P, Rahimi M, Adibi H, Razmjou Z, Ghasempour H. An insight on reducing the particle size of poorly-water soluble curcumin via LASP in microchannels. Chem Eng Process Process Intensif. 2015;91:78–88.
Ramakers LAI, McGinty J, Beckmann W, Levilain G, Lee M, Wheatcroft H, et al. Investigation of metastable zones and induction times in glycine crystallization across three different antisolvents. Cryst Growth Des American Chemical Society. 2020;20:4935–44.
Wang X, Gillian JM, Kirwan DJ. Quasi-emulsion precipitation of pharmaceuticals. 1. Conditions for formation and crystal nucleation and growth behavior. Cryst Growth Des. 2006;6:2214–27.
Bhavna, Ahmad FJ, Mittal G, Jain GK, Malhotra G, Khar RK, et al. Nano-salbutamol dry powder inhalation: a new approach for treating broncho-constrictive conditions. Eur J Pharm Biopharm. 2009;71:282–91.
Hussain MN, Baeten S, Jordens J, Braeken L, Van Gerven T. Process intensified anti-solvent crystallization of o-aminobenzoic acid via sonication and flow. Chem Eng Process - Process Intensif. 2020;149:107823.
Gandhi NV, Deokate UA, Angadi SS. Development of nanonized nitrendipine and its transformation into nanoparticulate oral fast dissolving drug delivery system. AAPS PharmSciTech. 2021;22:1–15.
Lee J, Ashokkumar M, Kentish SE. Influence of mixing and ultrasound frequency on antisolvent crystallisation of sodium chloride. Ultrason Sonochem. 2014;21:60–8.
Cerdeira AM, Mazzotti M, Gander B. Miconazole nanosuspensions: influence of formulation variables on particle size reduction and physical stability. Int J Pharm. 2010;396:210–8.
Li M, Azad M, Davé R, Bilgili E. Nanomilling of drugs for bioavailability enhancement: a holistic formulation-process perspective. Pharmaceutics. 2016;8.
Trzenschiok H, Distaso M, Peukert W. A new approach for the stabilization of amorphous drug nanoparticles during continuous antisolvent precipitation. Chem Eng J. 2019;361:428–38.
Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–20.
Lacerda S de P, Espitalier F, Hoffart V, Ré MI. Liquid anti-solvent recrystallization to enhance dissolution of CRS 74, a new antiretroviral drug. Drug Dev Ind Pharm. Informa Healthcare; 2015;41:1910–20.
Sartori GJ, Prado LD, Rocha HVA. Efavirenz dissolution enhancement IV—antisolvent nanocrystallization by sonication, physical stability, and dissolution. AAPS PharmSciTech. 2017;18:3011–20.
Kumar R, Siril PF. Drop-by-drop solvent hot antisolvent interaction method for engineering nanocrystallization of sulfamethoxazole to enhanced water solubility and bioavailability. J Drug Deliv Sci Technol. 2020;55:101359.
Prasad R, Dalvi SV. Understanding morphological evolution of griseofulvin particles into hierarchical microstructures during liquid antisolvent precipitation. Cryst Growth Des. 2019;19:5836–49.
Rangaraj N, Pailla SR, Chowta P, Sampathi S. Fabrication of ibrutinib nanosuspension by quality by design approach: intended for enhanced oral bioavailability and diminished fast fed variability. AAPS PharmSciTech. 2019;20:1–18.
Pandey KU, Poornachary SK, Dalvi SV. Insights to the action of additives for stabilization of ultrafine particles of Fenofibrate in aqueous suspensions produced by sonoprecipitation. Powder Technol. 2020;363:310–25.
Kim DC, Yeo S Do. Habit modification of tamoxifen crystals using antisolvent crystallizations. Korean J Chem Eng. 2017;34:1466–74.
Kuk DH, Ha ES, Ha DH, Sim WY, Lee SK, Jeong JS, et al. Development of a resveratrol nanosuspension using the antisolvent precipitation method without solvent removal, based on a quality by design (QbD) approach. Pharmaceutics. 2019;11:688.
Afrose A, White ET, Howes T, George G, Rashid A, Rintoul L, et al. Preparation of ibuprofen microparticles by antisolvent precipitation crystallization technique: characterization, formulation, and in vitro performance. J Pharm Sci. 2018;107:3060–9.
Wada S, Kudo S, Takiyama H. Development of simultaneous control of polymorphism and morphology in indomethacin crystallization. J Cryst Growth. 2016;435:37–41.
Thorat AA, Dalvi SV. Ultrasound-assisted modulation of concomitant polymorphism of curcumin during liquid antisolvent precipitation. Ultrason Sonochem. 2016;30:35–43.
Mohapatra PK, Sireesha, Rathore V, Verma HC, Bibhuti, Rath P, et al. Fabrication and in vitro characterization of a novel nanosuspension of telmisartan: a poorly soluble drug prepared by antisolvent precipitation technique using 33 factorial design. Int J Appl Pharm. 2020;12:286–94.
Alshweiat A, Csóka IiI, Tömösi F, Janáky T, Kovács A, Gáspár R, et al. Nasal delivery of nanosuspension-based mucoadhesive formulation with improved bioavailability of loratadine: preparation, characterization, and in vivo evaluation. Int J Pharm. 2020;579:119166.
Aly UF, Sarhan HAM, Ali TFS, Sharkawy HAEB. Applying different techniques to improve the bioavailability of candesartan cilexetil antihypertensive drug. Drug Des Devel Ther. 2020;14:1851–65.
Li W, Zhao X, Sun X, Zu Y, Liu Y, Ge Y. Evaluation of antioxidant ability in vitro and bioavailability of trans -cinnamic acid nanoparticle by liquid antisolvent precipitate. J Nanomater. 2016;2016.
Bodnar K, Hudson SP, Rasmuson ÅC. Stepwise use of additives for improved control over formation & stability of mefenamic acid nanocrystals produced by antisolvent precipitation. Cryst Growth Des. 2017;17:454–66.
Tierney TB, Guo Y, Beloshapkin S, Rasmuson ÅC, Hudson SP. Investigation of the particle growth of fenofibrate following antisolvent precipitation and freeze-drying. Cryst Growth Des. 2015;15:5213–22.
Dalvi SV, Yadav MD. Effect of ultrasound and stabilizers on nucleation kinetics of curcumin during liquid antisolvent precipitation. Ultrason Sonochem. 2015;24:114–22.
Hao J, Gao Y, Zhao J, Zhang J, Li Q, Zhao Z, et al. Preparation and optimization of resveratrol nanosuspensions by antisolvent precipitation using box-behnken design. AAPS PharmSciTech. 2014;16:118–28.
Shariare MH, Altamimi MA, Marzan AL, Tabassum R, Jahan B, Reza HM, et al. In vitro dissolution and bioavailability study of furosemide nanosuspension prepared using design of experiment (DoE). Saudi Pharm J. 2019;27:96–105.
Rao Q, Qiu Z, Huang D, Lu T, Zhang ZJ, Luo D, et al. Enhancement of the apparent solubility and bioavailability of tadalafil nanoparticles via antisolvent precipitation. Eur J Pharm Sci. 2019;128:222–31.
Rahim H, Sadiq A, Khan S, Amin F, Ullah R, Shahat AA, et al. Fabrication and characterization of glimepiride nanosuspension by ultrasonication-assisted precipitation for improvement of oral bioavailability and in vitro α-glucosidase inhibition. Int J Nanomedicine. 2019;14:6287–96.
Wu M, Feng Z, Deng Y, Zhong C, Liu Y, Liu J, et al. Liquid antisolvent precipitation: an effective method for ocular targeting of lutein esters. Int J Nanomedicine. 2019;14:2667–81.
Hu TT, Zhao H, Jiang LC, Le Y, Chen JF, Yun J. Engineering pharmaceutical fine particles of budesonide for dry powder inhalation (DPI). Ind Eng Chem Res. 2008;47:9623–7.
Resende de Azevedo J. Ultrasound assisted crystallization of a new cardioactive prototype using ionic liquid as solvent. Ultrason Sonochem. 2019;55:32–43.
Prasad R, Panwar K, Katla J, Dalvi SV. Polymorphism and particle formation pathway of carbamazepine during sonoprecipitation from ionic liquid solutions. Cryst Growth Des. 2020;20:5169–83.
Grodowska K, Parczewski A. Organic solvents in the pharmaceutical industry. Acta Pol Pharm - Drug Res. 2010;67:3–12.
Resende de Azevedo J, Jean-Jacques L, Espitalier F, Ré MI. Solubility of a new cardioactive prototype drug in ionic liquids. J Chem Eng Data. 2014;59:1766–73.
Pedro SN, Freire CSR, Silvestre AJD, Freire MG. The role of ionic liquids in the pharmaceutical field: an overview of relevant applications. Int J Mol Sci. 2020;1–50.
Kunov-Kruse AJ, Weber CC, Rogers RD, Myerson AS. The a priori design and selection of ionic liquids as solvents for active pharmaceutical ingredients. Chem - A Eur J. 2017;23:5498–508.
Marrucho IM, Branco LC, Rebelo LPN. Ionic liquids in pharmaceutical applications. Annu Rev Chem Biomol Eng. 2014;5:527–46.
Adawiyah N, Moniruzzaman M, Hawatulaila S, Goto M. Ionic liquids as a potential tool for drug delivery systems. MedChemComm. 2016;7(10):1881–97. https://doi.org/10.1039/C6MD00358C.
Yang Q, Zu C, Li W, Wu W, Ge Y, Wang L, et al. Enhanced water solubility and oral bioavailability of paclitaxel crystal powders through an innovative antisolvent precipitation process: antisolvent crystallization using ionic liquids as solvent. Pharmaceutics. 2020;12:1–16.
An JH, Jin F, Kim HS, Ryu HC, Kim JS, Kim HM, et al. Investigation of the polymorphic transformation of the active pharmaceutical ingredient clopidogrel bisulfate using the ionic liquid AEImBF4. Cryst Growth Des. 2016;16:1829–36.
An JH, Kim WS. Antisolvent crystallization using ionic liquids as solvent and antisolvent for polymorphic design of active pharmaceutical ingredient. Cryst Growth Des. 2013;13:31–9.
Viçosa A, Letourneau JJ, Espitalier F, Inês Ré M. An innovative antisolvent precipitation process as a promising technique to prepare ultrafine rifampicin particles. J Cryst Growth. 2012;80–7.
Sahibzada MUK, Zahoor M, Sadiq A, ur Rehman F, Al-Mohaimeed AM, Shahid M, et al. Bioavailability and hepatoprotection enhancement of berberine and its nanoparticles prepared by liquid antisolvent method. Saudi J Biol Sci. 2021;28:327–32.
Gera S, Pooladanda V, Godugu C, Swamy Challa V, Wankar J, Dodoala S, et al. Rutin nanosuspension for potential management of osteoporosis: effect of particle size reduction on oral bioavailability, in vitro and in vivo activity. Pharm Dev Technol. 2020;25:971–88.
Wu W, Wang L, Wang L, Zu Y, Wang S, Liu P, et al. Preparation of honokiol nanoparticles by liquid antisolvent precipitation technique, characterization, pharmacokinetics, and evaluation of inhibitory effect on HepG2 cells. Int J Nanomedicine. 2018;13:5469–83.
Neerati P, Palle S. Resveratrol nanoparticle pretreatment improved the oral bioavailability of bromocriptine: involvement of liver and intestinal CYP3A enzyme inhibition. J Nat Sci Biol Med. 2019;10:209–16.
Wang Z, Zhao X, Zu Y, Wu W, Li Y, Guo Z, et al. Licorice flavonoids nanoparticles prepared by liquid antisolvent re-crystallization exhibit higher oral bioavailability and antioxidant activity in rat. J Funct Foods. 2019;57:190–201.
Wang L, Zhao X, Yang F, Wu W, Liu Y, Wang L, et al. Enhanced bioaccessibility in vitro and bioavailability of ginkgo biloba extract nanoparticles prepared by liquid anti-solvent precipitation. Int J Food Sci Technol. 2019;54:2266–76.
Sahibzada MUK, Sadiq A, Zahoor M, Naz S, Shahid M, Qureshi NA. Enhancement of bioavailability and hepatoprotection by silibinin through conversion to nanoparticles prepared by liquid antisolvent method. Arab J Chem. 2020;13:3682–9.
Som S, Singh SK, Khatik GL, Kapoor B, Gulati M, Kuppusamy G, et al. Quality by design-based crystallization of curcumin using liquid antisolvent precipitation: micromeritic, biopharmaceutical, and stability aspects. Assay Drug Dev Technol. 2020;18:11–33.
Zhao X, Wang W, Zu Y, Zhang Y, Li Y, Sun W, et al. Preparation and characterization of betulin nanoparticles for oral hypoglycemic drug by antisolvent precipitation. Drug Deliv. 2014;21:467–79.
Pandey KU, Dalvi SV. Understanding stability relationships among three curcumin polymorphs. Adv Powder Technol. 2019;30:266–76.
Wu W, Zu Y, Wang L, Wang L, Wang H, Li Y, et al. Preparation, characterization and antitumor activity evaluation of apigenin nanoparticles by the liquid antisolvent precipitation technique. Drug Deliv. 2017;24:1713–20.
Lei Y, Kong Y, Sui H, Feng J, Zhu R, Wang W. Enhanced oral bioavailability of glycyrrhetinic acid via nanocrystal formulation. Drug Deliv Transl Res. 2016;6:519–25.
Wu W, Zu Y, Wang L, Wang L, Li Y, Liu Y, et al. Preparation, characterization and antitumor activity evaluation of silibinin nanoparticles for oral delivery through liquid antisolvent precipitation. RSC Adv. 2017;7:54379–90.
Joye IJ, Davidov-Pardo G, McClements DJ. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 2: stability and functionality. Food Hydrocoll. 2015;49:127–34.
Sun CC, Su H, Zheng GD, Wang WJ, Yuan E, Zhang QF. Fabrication and characterization of dihydromyricetin encapsulated zein-caseinate nanoparticles and its bioavailability in rat. Food Chem. 2020;330:127245.
Zheng D, Zhang QF. Bioavailability enhancement of astilbin in rats through zein− caseinate nanoparticles. J Agric Food Chem. 2019;67:5746–53.
Davidov-Pardo G, Joye IJ, McClements DJ. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 1: preparation and characterization. Food Hydrocoll. 2015;45:309–16.
Zhang X, Zhang H, Xia X, Pu N, Yu Z, Nabih M, et al. Preparation and physicochemical characterization of soy isoflavone (SIF) nanoparticles by a liquid antisolvent precipitation method. Adv Powder Technol. 2019;30:1522–30.
Ramisetty KA, Pandit AB, Gogate PR. Ultrasound-assisted antisolvent crystallization of benzoic acid: effect of process variables supported by theoretical simulations. Ind Eng Chem Res. 2013;52:17573–82.
Lee SK, Sim WY, Ha ES, Park H, Kim JS, Jeong JS, et al. Solubility of bisacodyl in fourteen mono solvents and N-methyl-2-pyrrolidone + water mixed solvents at different temperatures, and its application for nanosuspension formation using liquid antisolvent precipitation. J Mol Liq. 2020;310.
Xia D, Cui F, Piao H, Cun D, Piao H, Jiang Y, et al. Effect of crystal size on the in vitro dissolution and oral absorption of nitrendipine in rats. Pharm Res. 2010;27:1965–76.
Meer TA, Sawant KP, Amin PD. Liquid antisolvent precipitation process for solubility modulation of bicalutamide. Acta Pharm. 2011;61:435–45.
Zu Y, Li N, Zhao X, Li Y, Ge Y, Wang W, et al. In vitro dissolution enhancement of micronized l-nimodipine by antisolvent re-crystallization from its crystal form H. Int J Pharm. 2014;464:1–9.
Deshpande RD, Gowda DV, Vegesna NSKV, Vaghela R, Kulkarni PK. The effect of nanonization on poorly water soluble glibenclamide using a liquid anti-solvent precipitation technique: aqueous solubility, in vitro and in vivo study. RSC Adv. 2015;5:81728–38.
Kim HJ, Yeo SD. Liquid antisolvent crystallization of griseofulvin from organic solutions. Chem Eng Res Des. 2015;97:68–76.
Matteucci ME, Hotze MA, Johnston KP, Williams RO. Drug nanoparticles by antisolvent precipitation: mixing energy versus surfactant stabilization. Langmuir. 2006;22:8951–9.
Zhang ZB, Shen ZG, Wang JX, Zhao H, Chen JF, Yun J. Nanonization of megestrol acetate by liquid precipitation. Ind Eng Chem Res. 2009;48:8493–9.
Zu Y, Sun W, Zhao X, Wang W, Li Y, Ge Y, et al. Preparation and characterization of amorphous amphotericin B nanoparticles for oral administration through liquid antisolvent precipitation. Eur J Pharm Sci. 2014;53:109–17.
Zhao H, Wang JX, Wang QA, Chen JF, Yun J. Controlled liquid antisolvent precipitation of hydrophobic pharmaceutical nanoparticles in a MicroChannel reactor. Ind Eng Chem Res. 2007;46:8229–35.
Zhang Z, Ji J. Large-scale preparation of stable irbesartan nanoparticles by high-gravity liquid antisolvent precipitation technique. Powder Technol. 2017;305:546–52.
Park SJ, Yeo SD. Liquid antisolvent recrystallization of phenylbutazone and the effect of process parameters. Sep Sci Technol. 2011;46:1273–9.
Joye IJ, Nelis VA, McClements DJ. Gliadin-based nanoparticles: fabrication and stability of food-grade colloidal delivery systems. Food Hydrocoll. 2015;44:86–93.
Kakran M, Sahoo NG, Tan IL, Li L. Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J Nanoparticle Res. 2012;14:1–11.
Rathod WR, Rathod VK. Continuous preparation of nimesulide nanoparticles by liquid antisolvent precipitation using spinning disc reactor. J Chem Technol Biotechnol. 2019;94:919–26.
Shah SR, Parikh RH, Chavda JR, Sheth NR. Application of Plackett-Burman screening design for preparing glibenclamide nanoparticles for dissolution enhancement. Powder Technol. 2013;235:405–11.
Mishra B, Sahoo J, Dixit PK. Enhanced bioavailability of cinnarizine nanosuspensions by particle size engineering: optimization and physicochemical investigations. Mater Sci Eng C. 2016;63:62–9.
Zhu WZ, Wang JX, Shao L, Zhang HX, Zhang QX, Chen JF. Liquid antisolvent preparation of amorphous cefuroxime axetil nanoparticles in a tube-in-tube microchannel reactor. Int J Pharm. 2010;395:260–5.
Roy S, Bachchhav SD, Mukhopadhyay M. Analysis of the mechanism of cholesterol particle formation by liquid antisolvent crystallization. Ind Eng Chem Res. 2021;60:7975–86.
Park SJ, Jeon SY, Yeo SD. Recrystallization of a pharmaceutical compound using liquid and supercritical antisolvents. Ind Eng Chem Res. 2006;45:2287–93.
Chen J, Sarma B, Evans JMB, Myerson AS. Pharmaceutical crystallization. Cryst Growth Des. 2011;11:887–95.
Leuenberger H. New trends in the production of pharmaceutical granules: batch versus continuous processing. Eur J Pharm Biopharm. 2001;289–96.
Benitez-Chapa AG, Nigam KDP, Alvarez AJ. Process intensification of continuous antisolvent crystallization using a coiled flow inverter. Ind Eng Chem Res. 2020;59:3934–42.
Wang J, Lakerveld R. Integrated solvent and process design for continuous crystallization and solvent recycling using PC-SAFT. AIChE J. 2018;64:1205–16.
Orehek J, Češnovar M, Teslić D, Likozar B. Mechanistic crystal size distribution (CSD)-based modelling of continuous antisolvent crystallization of benzoic acid. Chem Eng Res Des. 2021;170:256–69.
Solymosi T, Angi R, Basa-Dénes O, Ránky S, Ötvös Z, Glavinas H, et al. Sirolimus formulation with improved pharmacokinetic properties produced by a continuous flow method. Eur J Pharm Biopharm. 2015;94:135–40.
Hussain MN, Jordens J, John JJ, Braeken L, Van Gerven T. Enhancing pharmaceutical crystallization in a flow crystallizer with ultrasound: Anti-solvent crystallization. Ultrason Sonochem. 2019;59:104743.
Vancleef A, Seurs S, Jordens J, Van Gerven T, Thomassen LCJ, Braeken L. Reducing the induction time using ultrasound and high-shear mixing in a continuous crystallization process. Curr Comput-Aided Drug Des. 2018;8:326.
Davey RJ, Back KR, Sullivan RA. Crystal nucleation from solutions - transition states, rate determining steps and complexity. Faraday Discuss. 2015;9–26.
Ferguson S, Morris G, Hao H, Barrett M, Glennon B. In-situ monitoring and characterization of plug flow crystallizers. Chem Eng Sci. 2012;77:105–11.
Azad MA, Knieke C, To D, Davé R. Preparation of concentrated stable fenofibrate suspensions via liquid antisolvent precipitation. Drug Dev Ind Pharm. 2014;40:1693–703.
Rahimi M, Valeh-e-Sheyda P, Parsamoghadam MA, Azimi N, Abidi H. LASP and Villermaux/Dushman protocols for mixing performance in microchannels: effect of geometry on micromixing characterization and size reduction. Chem Eng Process Process Intensif. 2014;85:178–86.
Valeh-e-Sheyda P, Rahimi M, Parsamoghadam A, Adibi H. Effect of microchannel confluence angles on size reduction of curcumin nano-suspension via liquid anti-solvent precipitation process. J Taiwan Inst Chem Eng. 2015;46:65–73.
Shrimal P, Jadeja G, Patel S. Microfluidics nanoprecipitation of telmisartan nanoparticles: effect of process and formulation parameters. Chem Pap. 2021;75:205–14.
Chen H, Zhang X, Cheng Y, Qian F. Preparation of smectic itraconazole nanoparticles with tunable periodic order using microfluidics-based anti-solvent precipitation. CrystEngComm. 2019;21:2362–72.
Le NHA, Van PH, Yu J, Chan HK, Neild A, Alan T. Acoustically enhanced microfluidic mixer to synthesize highly uniform nanodrugs without the addition of stabilizers. Int J Nanomed. 2018;13:1353–9.
Rahimi M, Valeh-e-Sheyda P, Zarghami R, Rashidi H. On the mixing characteristics of a poorly water soluble drug through microfluidic-assisted nanoprecipitation: experimental and numerical study. Can J Chem Eng. 2018;96:1098–108.
Hu J, Ng WK, Dong Y, Shen S, Tan RBH. Continuous and scalable process for water-redispersible nanoformulation of poorly aqueous soluble APIs by antisolvent precipitation and spray-drying. Int J Pharm. 2011;404:198–204.
Hu J, Dong Y, Ng WK, Pastorin G. Preparation of drug nanocrystals embedded in mannitol microcrystals via liquid antisolvent precipitation followed by immediate (on-line) spray drying. Adv Powder Technol. 2018;29:957–63.
Dong Y, Ng WK, Hu J, Shen S, Tan RBH. Clay as a matrix former for spray drying of drug nanosuspensions. Int J Pharm. 2014;465:83–9.
Gu C, Liu Z, Yuan X, Li W, Zu Y, Fu Y. Preparation of vitexin nanoparticles by combining the antisolvent precipitation and high pressure homogenization approaches followed by lyophilization for dissolution rate enhancement. Molecules. 2017;22:2038.
Zhang J, Lv H, Jiang K, Gao Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm. 2011;420:180–8.
Xu LM, Zhang QX, Zhou Y, Zhao H, Wang JX, Chen JF. Engineering drug ultrafine particles of beclomethasone dipropionate for dry powder inhalation. Int J Pharm. 2012;436:1–9.
Kumar S, Shen J, Burgess DJ. Nano-amorphous spray dried powder to improve oral bioavailability of itraconazole. J Control Release. 2014;192:95–102.
Ma Q, Sun H, Che E, Zheng X, Jiang T, Sun C, et al. Uniform nano-sized valsartan for dissolution and bioavailability enhancement: influence of particle size and crystalline state. Int J Pharm. 2013;441:75–81.
Zhong J, Shen Z, Yang Y, Chen J. Preparation and characterization of uniform nanosized cephradine by combination of reactive precipitation and liquid anti-solvent precipitation under high gravity environment. Int J Pharm. 2005;301:286–93.
Pandey NK, Singh SK, Gulati M, Kumar B, Kapoor B, Ghosh D, et al. Overcoming the dissolution rate, gastrointestinal permeability and oral bioavailability of glimepiride and simvastatin co-delivered in the form of nanosuspension and solid self-nanoemulsifying drug delivery system: a comparative study. J Drug Deliv Sci Technol. 2020;60:102083.
Mahesh KV, Singh SK, Gulati M. A comparative study of top-down and bottom-up approaches for the preparation of nanosuspensions of glipizide. Powder Technol. 2014;256:436–49.
Zhang H, Meng Y, Wang X, Dai W, Wang X, Zhang Q. Pharmaceutical and pharmacokinetic characteristics of different types of fenofibrate nanocrystals prepared by different bottom-up approaches. Drug Deliv. 2014;21:588–94.
Bolourchian N, Nili M, Foroutan SM, Mahboubi A, Nokhodchi A. The use of cooling and anti-solvent precipitation technique to tailor dissolution and physicochemical properties of meloxicam for better performance. J Drug Deliv Sci Technol. 2020;55:101485.
Yeo SD, Lee JC. Crystallization of sulfamethizole using the supercritical and liquid antisolvent processes. J Supercrit Fluids. 2004;30:315–23.
Wu W, Zu Y, Zhao X, Zhang X, Wang L, Li Y, et al. Solubility and dissolution rate improvement of the inclusion complex of apigenin with 2-hydroxypropyl-β-cyclodextrin prepared using the liquid antisolvent precipitation and solvent removal combination methods. Drug Dev Ind Pharm. 2017;43:1366–77.
Pandey KU, Joshi A, Dalvi SV. Evaluating the efficacy of different curcumin polymorphs in transdermal drug delivery. J Pharm Investig. 2021;51:75–84.
Kedia K, Wairkar S. Improved micromeritics, packing properties and compressibility of high dose drug, cycloserine, by spherical crystallization. Powder Technol. 2019;344:665–72.
Azad MA, Sievens-Figueroa L, Davé RN. Fast release of liquid antisolvent precipitated fenofibrate at high drug loading from biocompatible thin films. Adv Powder Technol. 2018;29:2907–19.
Chandra A, Chondkar AD, Shirodkar R, Lewis SA. Rapidly dissolving lacidipine nanoparticle strips for transbuccal administration. J Drug Deliv Sci Technol. 2018;47:259–67.
Beck C, Sievens-Figueroa L, Gärtner K, Jerez-Rozo JI, Romañach RJ, Bilgili E, et al. Effects of stabilizers on particle redispersion and dissolution from polymer strip films containing liquid antisolvent precipitated griseofulvin particles. Powder Technol. 2013;236:37–51.
Wu W, Wang L, Wang S. Amorphous silibinin nanoparticles loaded into porous starch to enhance remarkably its solubility and bioavailability in vivo. Colloids Surfaces B Biointerfaces. 2021;198:111474
Joye IJ, McClements DJ. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci Technol. 2013:109–23.
Langer K, Balthasar S, Vogel V, Dinauer N, Von Briesen H, Schubert D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm. 2003;257:169–80.
Khan SA, Schneider M. Improvement of nanoprecipitation technique for preparation of gelatin nanoparticles and potential macromolecular drug loading. Macromol Biosci. 2013;13:455–63.
Acknowledgements
The authors acknowledge their respective organizations for permitting to do this work.
Author information
Authors and Affiliations
Contributions
Dr. Rahul Kumar took a lead in writing this manuscript. Dr. Amit K. Thakur wrote the nanonization of herbal drugs. Dr. Nilanjana Banerjee revised the section “Comparison of LASC and other techniques.” Dr. Ashutosh Kumar wrote the combinative approach. Dr. Gajendra Kumar Gaurav edited the original draft of manuscript. Dr. Nilanjana Banerjee and Dr. Raj Kumar Arya did editing of the revised version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Thakur, A.K., Banerjee, N. et al. Liquid antisolvent crystallization of pharmaceutical compounds: current status and future perspectives. Drug Deliv. and Transl. Res. 13, 400–418 (2023). https://doi.org/10.1007/s13346-022-01219-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01219-1