Abstract
Background
Severe congenital anomalies of the kidney and urinary tract (CAKUT) progress to infantile kidney failure with replacement therapy (KFRT). Although prompt and precise prediction of kidney outcomes is important, early predictive factors for its progression remain incompletely defined.
Methods
This retrospective cohort study included patients with CAKUT treated at 12 centers between 2009 and 2020. Patients with a maximum serum creatinine level ≤ 1.0 mg/dL during the first 3 days, patients who died of respiratory failure during the neonatal period, patients who progressed to KFRT within the first 3 days, and patients lacking sufficient data were excluded.
Results
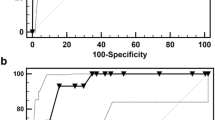
Of 2187 patients with CAKUT, 92 were finally analyzed. Twenty-five patients (27%) progressed to KFRT and 24 (26%) had stage 3–5 chronic kidney disease without replacement therapy during the median observation period of 52.0 (interquartile range, 22.0–87.8) months. Among these, 22 (24%) progressed to infantile KFRT. The kidney survival rate during the infantile period was significantly lower in patients with a maximum serum creatinine level during the first 3 days (Cr-day3-max) ≥ 2.5 mg/dL (21.8%) compared with those with a Cr-day3-max < 2.5 mg/dL (95.2%) (log-rank, P < 0.001). Multivariate analysis demonstrated Cr-day3-max (P < 0.001) and oligohydramnios (P = 0.025) were associated with higher risk of infantile KFRT. Eighty-two patients (89%) were alive at the last follow-up.
Conclusions
Neonatal kidney function, including Cr-day3-max, was associated with kidney outcomes in patients with severe CAKUT. Aggressive therapy for severe CAKUT may have good long-term life outcomes through infantile dialysis and kidney transplantation.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information



Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Ichikawa I, Kuwayama F, Pope JC 4th, Stephens FD, Miyazaki Y (2020) Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT. Kidney Int 61:889–898. https://doi.org/10.1046/j.1523-1755.2002.00188.x
Murugapoopathy V, Gupta IR (2020) A primer on congenital anomalies of the kidneys and urinary tracts (CAKUT). Clin J Am Soc Nephrol 15:723–731. https://doi.org/10.2215/CJN.12581019
Ishikura K, Uemura O, Ito S, Wada N, Hattori M, Ohashi Y, Hamasaki Y, Tanaka R, Nakanishi K, Kaneko T, Honda M, Pediatric CKD Study Group; Japan Committee of Measures for Pediatric CKD of the Japanese Society of Pediatric Nephrology (2013) Pre-dialysis chronic kidney disease in children: results of a nationwide survey in Japan. Nephrol Dial Transplant 28:2345–2355. https://doi.org/10.1093/ndt/gfs611
Hattori M, Sako M, Kaneko T, Ashida A, Matsunaga A, Igarashi T, Itami N, Ohta T, Gotoh Y, Satomura K, Honda M, Igarashi T (2015) End-stage renal disease in Japanese children: a nationwide survey during 2006–2011. Clin Exp Nephrol 19:933–938. https://doi.org/10.1007/s10157-014-1077-8
van Stralen KJ, Borzych-Dużalka D, Hataya H, Kennedy SE, Jager KJ, Verrina E, Inward C, Rönnholm K, Vondrak K, Warady BA, Zurowska AM, Schaefer F, Cochat P, ESPN/ERA-EDTA registry, IPPN registry, ANZDATA registry, Japanese RRT registry (2014) Survival and clinical outcomes of children starting renal replacement therapy in the neonatal period. Kidney Int 86:168–174. https://doi.org/10.1038/ki.2013.561
Zurowska AM, Fischbach M, Watson AR, Edefonti A, Stefanidis CJ, European Paediatric Dialysis Working Group (2013) Clinical practice recommendations for the care of infants with stage 5 chronic kidney disease (CKD5). Pediatr Nephrol 28:1739–1748. https://doi.org/10.1007/s00467-012-2300-z
Katsoufis CP, DeFreitas MJ, Infante JC, Castellan M, Cano T, Safina Vaccaro D, Seeherunvong W, Chandar JJ, Abitbol CL (2019) Risk assessment of severe congenital anomalies of the kidney and urinary tract (CAKUT): a birth cohort. Front Pediatr 7:182. https://doi.org/10.3389/fped.2019.00182
Carey WA, Martz KL, Warady BA (2015) Outcome of patients initiating chronic peritoneal dialysis during the first year of life. Pediatrics 136:e615–e622. https://doi.org/10.1542/peds.2015-0980
Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158:825–830. https://doi.org/10.7326/0003-4819-158-11-201306040-00007
Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, Fujita N, Akioka Y, Kaneko T, Honda M (2014) Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol 18:626–633. https://doi.org/10.1007/s10157-013-0856-y
Uemura O, Ishikura K, Gotoh Y, Honda M (2018) Creatinine-based estimated glomerular filtration rate for children younger than 2 years. Clin Exp Nephrol 22:483–484. https://doi.org/10.1007/s10157-017-1460-3
Jetton JG, Askenazi DJ (2012) Update on acute kidney injury in the neonate. Curr Opin Pediatr 24:191–196. https://doi.org/10.1097/MOP.0b013e32834f62d5
González Celedón C, Bitsori M, Tullus K (2007) Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol 22:1014–1020. https://doi.org/10.1007/s00467-007-0459-5
Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, Izzi C, Scolari F, Mattioli G, Allegri L, Ghiggeri GM (2009) Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int 76:528–533. https://doi.org/10.1038/ki.2009.220
Quirino IG, Diniz JS, Bouzada MC, Pereira AK, Lopes TJ, Paixão GM, Barros NN, Figueiredo LC, Cabral AC, Simões e Silva AC, Oliveira EA (2012) Clinical course of 822 children with prenatally detected nephrouropathies. Clin J Am Soc Nephrol 7:444–451. https://doi.org/10.2215/CJN.03400411
Tsai TC, Chen YC, Lo CW, Wang WS, Lo SS, Tang GJ, Thien PF (2014) Incidence and renal survival of ESRD in the young Taiwanese population. Clin J Am Soc Nephrol 9:302–309. https://doi.org/10.2215/CJN.12761212
Suzuki H, Suzuki K (1995) Pathophysiology and postnatal pathogenesis of hypoplastic kidney (hpk/hpk) in the male hypogonadic mutant rat (hgn/hgn). J Vet Med Sci 57:891–897. https://doi.org/10.1292/jvms.57.891
Nef S, Neuhaus TJ, Spartà G, Weitz M, Buder K, Wisser J, Gobet R, Willi U, Laube GF (2016) Outcome after prenatal diagnosis of congenital anomalies of the kidney and urinary tract. Eur J Pediatr 175:667–676. https://doi.org/10.1007/s00431-015-2687-1
Mansoor O, Chandar J, Rodriguez MM, Abitbol CL, Seeherunvong W, Freundlich M, Zilleruelo G (2011) Long-term risk of chronic kidney disease in unilateral multicystic dysplastic kidney. Pediatr Nephrol 26:597–603. https://doi.org/10.1007/s00467-010-1746-0
Potter EL (1946) Facial characteristics of infants with bilateral renal agenesis. Am J Obstet Gynecol 51:885–888. https://doi.org/10.1016/s0002-9378(16)39968-9
Sarkar S, DasGupta S, Barua M, Ghosh R, Mondal K, Chatterjee U, Datta C (2015) Potter’s sequence: a story of the rare, rarer and the rarest. Indian J Pathol Microbiol 58:102–104. https://doi.org/10.4103/0377-4929.151202
Acknowledgements
The authors would like to thank Dr. Naoya Morisada for conducting the genetic diagnosis; Drs. Hitoshi Yoda, Norio Mizukaki, and Mai Kubota for providing clinical data; and Dr. Shuichi Ito for their overall contributions to the study. The authors also thank the medical editors from the Division of Education for Clinical Research at the National Center for Child Health and Development for editing a draft of this manuscript. We also thank Susan Furness, PhD, and J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This work was funded by childhood-onset, rare, and intractable kidney diseases in Japan, research on rare and intractable diseases, Health, Labour and Welfare Sciences Research Grants (20FC1028).
Author information
Authors and Affiliations
Consortia
Contributions
KN prepared the first draft of the manuscript, oversaw the data collection, and performed the data analysis as the primary investigator. RHar, MY, YO, KM, YG, TKi, DH, YH, NF, TU, TN, TI, and RHam performed the research, and edited and reviewed the manuscript. TKa designed the study, analyzed the data, and revised the manuscript for important intellectual content. KK conducted the statistical analysis. OU supervised and designed the study and critically revised the manuscript. KI designed the study, critically revised the manuscript for important intellectual content, and oversaw the work as the corresponding author. All authors contributed to the study conception and design and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the institutional ethics committee of the National Center for Child Health and Development (approval no. 2020–169). Study approval with agreement for data was shared among each institution’s ethics committee.
Informed consent
Informed consent for participating in this study was not required in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects of the Ministry of Health, Labour, and Welfare. Consent for publication was waived in accordance with the guidelines.
Competing interests
Kenji Ishikura has received research funding from the Asahi Kasei Pharma Corporation, Novartis International AG, Japan Blood Products Organization, Teijin Pharma Limited, JCR Pharmaceuticals Co., Ltd., Chugai Pharmaceutical Co., Ltd., Zenyaku Kogyo Co., Ltd., Otsuka Pharmaceutical Co. Ltd., Shionogi Co., Ltd., and The Morinaga Foundation for Health & Nutrition; and lecture fees from Asahi Kasei Pharma Corporation, Novartis International AG, Chugai Pharmaceutical Co., Ltd., Zenyaku Kogyo Co., Ltd., Otsuka Pharmaceutical Co. Ltd., Teijin Pharma Limited, Astellas Pharma Inc., Sanofi S.A., Pfizer Inc., AstraZeneca plc, and Miyarisan Pharmaceutical Co. Yusuke Okuda has received research funding from JSPS Kakenhi. Yuko Hamasaki has received lecture fees from Torii Pharmaceutical Co., Ltd and Pfizer Inc. Koichi Kamei has received research funding from the Terumo Foundation for Life Sciences and Arts, Public Foundation of Vaccination Research Center, and Taiju Life Social Welfare Foundation; donations from Chugai Pharmaceutical Co. Ltd., Astellas Pharma Inc., Ono Pharmaceutical Co., Teijin Pharma Ltd., Shionogi Co. Ltd., and Otsuka Pharmaceutical Co. Ltd.; and lecture fees from Tanabe Mitsubishi Pharma, Baxter Ltd., and Zenyaku Kogyo Co. Ltd. Other authors have no potential conflicts of interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
467_2022_5703_MOESM2_ESM.jpg
Supplementary file2 (JPG 2771 KB) Supplemental Figure Comparison of Cr values between the infantile KFRT group and non-KF/older KFRT group. Comparison of (A) Cr-day1-max, (B) Cr-day2-max, (C) Cr-day3-max, (D) Cr-day4-max, (E) Cr-day5-max, (F) Cr-day6-max, (G) Cr-day7-max, and (H) Peak SCr between groups. Peak SCr was defined as maximum value of SCr during neonatal period before initiation of dialysis. Cr-day1-max, maximum serum creatinine level during the first 1 day; Cr-day2-max, maximum serum creatinine level during the first 2 days; Cr-day3-max, maximum serum creatinine level during the first 3 days; Cr-day4-max, maximum serum creatinine level during the first 4 days; Cr-day5-max, maximum serum creatinine level during the first 5 days; Cr-day6-max, maximum serum creatinine level during the first 6 days; Cr-day7-max, maximum serum creatinine level during the first 7 days; I-KFRT, infantile kidney failure with replacement therapy; KFRT, kidney failure with replacement therapy; non-KF, non-kidney failure
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nishi, K., Uemura, O., Harada, R. et al. Early predictive factors for progression to kidney failure in infants with severe congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 38, 1057–1066 (2023). https://doi.org/10.1007/s00467-022-05703-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05703-1