Abstract
To investigate the use of biomass in the novel HIsarna technology, the reduction of FeO in the slag by chars produced from thermal coal (TC), charcoal (CC), and Bana grass char (BGC) was studied. A drop tube furnace coupled with a quadrupole mass spectrometer (DTF-QMS) was employed to study the injection of chars into pre-melted slag in the temperature range between 1450 °C and 1525 °C. The reduction rate was calculated from evolved gases and the extent of FeO reduction was confirmed by wavelength-dispersive X-ray fluorescence (WDXRF). The FeO reduction proceeds through two stages, starting with a rapid reduction, which is dependent on the carbon type, and followed by gradual leveling off. The reduction rate with the charcoal char (CC) was the highest, over 60 pct reduction was achieved in the first 500 seconds at 1500 °C, while ~ 50 and 40 pct achieved with TC and BGC chars, respectively, for the same reaction time. The kinetic analysis suggests that the first and second stages of the reaction can be described by the second-order (F2) and three-dimensional diffusion (D3) models, respectively. The apparent activation energy values for the first stage were 290, 229 and 267 kJ/mol for reactions with TC, CC and BGC chars, while 265, 369, and 282 kJ/mol were obtained for the second stage. Based on the experimental data and kinetic results, it can be concluded that the first stage is controlled by chemical reactions on the carbon surface, and the second stage is influenced by a mixed-controlling mechanism.
Similar content being viewed by others
Introduction
HIsarna represents a new route of smelting reduction process. It has been developed by combining two well-known technologies, i.e., the cyclone converter furnace (CCF) and the smelting reduction vessel (SRV), into a single highly integrated smelting furnace.[1,2] The process is a result of the European ULCOS (ultra-low CO2 steelmaking) program, to find an innovative solution for reducing CO2 emissions in steel manufacturing. The pilot plant located in Tata Steel, IJmuiden, the Netherlands, was constructed in 2010 with a capacity of 60,000 t/a. The process is flexible in raw materials, which can utilize low-cost thermal coal instead of coking coal and low-quality iron ore and potential for the use of complex iron ore (e.g., titanomagnetite).[3] By removing the coking, pelletizing, sintering, and blast furnace (BF) ironmaking steps, it reduces energy usage and CO2 emissions compared to the traditional BF process.[4,5] There have been many attempts to substitute coking coal and thermal coal (e.g., pulverized coal injection) with renewable biomass and natural gas in the BF, to reduce the environmental impacts and help improve sustainability. However, the necessity for burden support by coke has set limitations on using renewable biomass in the BF, which is not required in the HIsarna process. The flexibility of HIsarna process in raw materials creates the potential to substitute current thermal coal with low density and low strength biomass and still maintains the process efficiency and productivity.[6]
HIsarna starts with raw materials (i.e., iron ore and fluxing agents) and oxygen injected into the CCF, where CO-rich off-gas evolved from SRV is burned and reacts with the iron ore. The cyclone temperature can exceed 2000 °C, at which the iron ore melts and is partially reduced by up to 20 pct.[7,8] The molten iron ore forms a liquid film along the cyclone wall and falls down under gravity into the slag layer in the SRV where the temperature is between 1400 °C and 1500 °C. The granular thermal coal (or other carbonaceous materials) is injected into the slag layer in the SRV bath using nitrogen as a carrier gas to reduce iron oxide in slag to liquid metal and carburize the hot metal bath.[9] This injection process causes an intense metal–slag mixing and creates a large amount of splash of both metal and slag that circulate through the upper section of the SRV as droplets, where oxygen is introduced to generate heat by combustion of the upward gas stream. These droplets create a large metal–slag interfacial area and transfer heat from the post-combustion zone to the slag bath to maintain the temperature of the slag and metal.
It has been reported that the rate of reduction of iron oxide in the molten slag is controlled by dissolved carbon in hot metal (Fe–C) droplets and solid carbon.[10] Therefore, the knowledge and understanding of slag–carbon and carbon–metal reaction kinetics are critical aspects of the smelting process. The reduction of FeO containing slag by solid carbon is expressed by Eq. [1], which is considered as the overall reaction between FeO in the slag and solid carbon:
The reaction proceeds with the help of gaseous intermediates, as shown in Figure 1; when the reaction begins, a gas film is formed around the solid carbon which separates the molten slag from the carbon. The reduction process consists of five individual steps as described below[11,12,13]:
-
Chemical reactions at the slag–gas interface are expressed by Eq. [2]
$$ {\text{CO}}_{{\left( {\text{g}} \right)}} + {\text{ FeO}}_{{\left( {\text{l}} \right)}} \to {\text{Fe}}_{{\left( {\text{l}} \right)}} + {\text{CO}}_{{2\left( {\text{g}} \right)}} $$(2) -
Chemical reactions at the gas–carbon interface are expressed by Eq. [3]
$$ {\text{CO}}_{{2\left( {\text{g}} \right)}} + {\text{C}}_{{\left( {\text{s}} \right)}} \to {\text{2CO}}_{{\left( {\text{g}} \right)}} $$(3) -
Mass transfer of FeO from the bulk slag to the slag–gas interface
-
Carbon diffusion into liquid metal to form Fe–C
-
CO and CO2 gas diffusion through the gas halo
These steps are known to have an important effect on the reduction mechanism which controls the smelting performance. To understand the influence of each of the above steps in the reduction kinetics, several studies have been performed. The role of gas–carbon reaction (Eq. [3]) is studied by selecting different carbonaceous materials (graphite, coke, and coal char), and it is noted that different carbonaceous materials have different reactivity level toward CO2, which can be crucial in the CO generation.[10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] In the same studies, the effect of chemical reaction at slag–gas interface, rate of mass transfer of FeO in the liquid slag to slag–gas interface, and gas diffusion in the halo were analyzed to determine the rate-controlling step(s).
Sarma et al.[10] investigated the rate of carbon monoxide evolution from the reaction of slag containing 2 to 10 wt pct FeO with solid carbon (such as graphite, coke, and coal char) using stationary and rotating carbon rods, stationary horizontal carbon surfaces, and pinned stationary spheres as the reductants to determine the rate-controlling step.[10] It was found that the rate is controlled by liquid-phase mass transfer, gas diffusion, and chemical reaction at the slag–gas interface; therefore, the overall reduction rate is governed by a mixed-control mechanism. Seo and Fruehan[11] studied the reduction kinetics of blast furnace slags containing 1 to 3 wt pct FeO with a disk of coal char prepared from pulverized coal at 1450 °C. The finding indicates that the mixed-control mechanism is rate limiting but for low FeO content, liquid-phase mass transfer is the primary controlling step. The mass spectrometer technique was used by Min et al.[12] to measure CO and CO2 evolving from the reaction of graphite rod with slag containing a wide range of FeO content. It was observed that an increase in the FeO content has a positive effect on the rate of reduction. At low FeO (< 5 wt pct) content, the mass transfer of FeO in the liquid slag is rate limiting but at high FeO content (> 30 wt pct), chemical reaction at the gas–carbon interface is the rate-controlling step. Story et al.[13] re-examined the work done by Sarma et al.[10] for carbon gasification reaction using mixed-control model and found that the carbon type has a great influence on the reduction rate. Depending on the reaction conditions, i.e., agitation and carbon type, the overall rate is controlled by a mixed mechanism.
To determine the role of solid carbonaceous materials on the reduction of FeO in the slag, Teasdale and Hayes[18] reacted slag with graphite, coke, and two types of coal at the temperature range of 1400–1600 °C, using online mass spectrometry for real-time gas analysis and EPMA for quenched sample examination. The results suggest that the carbon type significantly affects the reaction rate, and carbon gasification takes place at the carbon surface; however, the slag phase mass transfer is defined as a rate-limiting step. In another study, Fruehan[20] studied the rate of reduction of iron oxide by chars produced from different biomass sources, coke, and coal at low temperature range between 900 °C and 1200 °C. Huang et al.[16] studied the interaction between slag containing iron oxide and a wide range of carbonaceous materials (including bio-chars, graphite, coke, and char from tire pyrolysis). It was found that the reaction takes place through a gaseous intermediate formed between solid char and iron oxide. The findings suggested that the carbon type has a significant influence on the reduction rate due to their interaction with the slag; therefore, carbon oxidation step is defined as a controlling mechanism. Various other factors have also been studied which may affect FeO reduction rate including particle size and quality of solid coal by Ozawa et al.[19] The results indicate that the rate is controlled by chemical reaction for high volatile matter coals, but for low volatile coals, liquid-phase mass transfer controls the rate. The finding by Ji et al.[23] for coal injection into electric arc furnace (EAF) slags reveals that the rate is controlled by chemical reactions at the slag and carbon interface. For high basicity slag greater than 3.2, the gas–carbon is the rate-limiting step but for slag basicity between 1.8 and 3.2, the reaction at both slag–gas and gas–carbon interfaces is likely to control the rate. However, for low slag basicity (< 1.8), gas–slag reaction becomes slower; therefore, it becomes the rate-limiting step. In another study, Ji et al.[24] used injection method to find the optimum conditions for coal injection into EAF slags for stablizing slag foaming. The study found that the carbon gasification is the main rate-limiting step but slag–gas reaction also has significant contribution during long injection runs. Results by Bafghi et al.[15] also indicate that the slag basicity controls the reduction mechanism, for the slag with a basicity of 2, the liquid-phase mass transfer is the rate limiting but for the lower basicity slag, the mixed-control model is applied. In another work by Bafghi et al.[14], the effect of slag foaming on reduction of FeO in the slag was studied. It was found the rate is affected by slag foaming and liquid-phase mass transfer is the rate-limiting step.
Additionally, other aspects including Fe–C droplets interaction with slag have been studied relevant to iron smelting process.[25,26,27,28,29] Molloseau and Fruehan[30] studied the reaction behavior of Fe–C–S droplets containing 2.9 pct C and 0.01 pct S with the slag containing 3 to 35 wt pct FeO in the temperature range of 1643 K to 1763 K. The study shows that the FeO content has significant effect on the droplet behavior and the droplets remained intact when the FeO content is less than 10 pct and the rate is controlled by chemical reaction on the metal surface. However, the increase in the FeO in slag causes the droplet to become emulsified within the slag which helps increase the rate of reaction. Smith and Fruehan[26] investigated the rate of reduction of FeO in the slag by carbon in the metal with different sulfur contents from moderate to high at 1723 K. It was found that at the high sulfur content, the rate was controlled by metal–gas reaction but mixed-controlled mechanism from FeO mass transfer and metal–gas is likely to control the rate for reaction with low sulfur content. In another study by Min and Fruehan,[27] it was confirmed that sulfur content has an important effect on the rate and at high sulfur content, the rate was controlled by kinetics at the metal–gas interface but for low sulfur content, the rate was controlled by liquid and gas mass transfer steps. The study by Biswas et al.[28] for Fe–C droplets with oxidizing slag suggests that the reaction is controlled by oxygen supply to the droplets; therefore, FeO liquid-phase mass transfer is the rate-limiting step. Gu et al.[29] conducted dephosphorization study by reacting iron droplets containing carbon as well as free from carbon with FeO-containing slag, and it was concluded that carbon content leads to faster dephosphorization rate due to the stirring effect from CO bubbles formed from reduction of FeO. A series of studies conducted by Barati and Coley[31,32,33] investigated the kinetics of the CO–CO2 reaction with iron oxide-containing slags. Through these studies, the authors developed a kinetic model to measure the rate of the CO–CO2 reaction with slag melt, which relates the apparent rate constant to temperature, and both pre-exponential and activation energy are functions of slag chemistry.
Among the researches reviewed here, there are some discrepancies in the rate-controlling step and mechanisms for the reaction between iron oxide containing slag and carbon from different sources. Limited information is available in the literature about the reaction kinetics for smelting reduction of FeO in the slag by solid carbon injection concerning HIsarna technology. Therefore, the purpose of this work is to investigate the reduction of FeO in synthetic HIsarna slag by injecting solid chars produced from different carbonaceous materials, i.e., thermal coal which is used in HIsarna trials and biomass which has potential to be trialed. To conduct this study, a technique using a drop tube furnace coupled with an online quadrupole mass spectrometer (DTF-QMS) was employed to study the reactions under thermal conditions similar to HIsarna SRV. The amount of CO and CO2 evolved from the reactions is measured using the online QMS to calculate FeO concentration in the slag and determine the overall reaction kinetics in the temperature range from 1450 °C to 1525 °C. The extent of FeO reduction in the reacted slag samples is also quantitatively measured by wavelength-dispersive X-ray fluorescence (WDXRF). Based on the experimental results, the kinetic parameters, the reaction mechanisms, and the rate-limiting steps for the reduction of FeO in the synthetic HIsarna slag are found for different carbonaceous materials.
Materials and Methods
Slag and Carbon Preparation
The synthetic slag with the weight composition of 41.7 pct CaO, 33.3 pct SiO2, 13 pct Al2O3, 6 pct MgO, and 6 pct FeO was prepared using reagent grade oxide powders (supplied by Sigma-Aldrich). The FeO powder used has purity ≥ 99.7 pct. The chemicals were mixed carefully to homogenize and then stored in well-sealed dry containers. Thermodynamic calculation by FactSage indicates that the first liquid slag forms at 1240 °C and the slag is fully molten at 1398 °C.
The solid chars used were prepared from three different carbon sources (one thermal coal and two biomass materials). The thermal coal (TC) and charcoal (CC) (a Birch wood-based pre-treated biomass) have already been used in HIsarna process during the pilot plant trials, while the Bana grass char (BGC) (derived from a grass-based torrefied material provided by Orange–Green through Tata Steel Nederland) is another renewable biomass source which may be considered for future HIsarna trials. All the samples were crushed into small particles with the size range from 90 to 300 μm, and then chars were prepared by heating each material to 1300 °C in high-purity (99.999 pct) argon atmosphere at the heating rate of 10 °C/min and held for 3 hours to ensure all the volatiles are removed. The proximate and ultimate analysis data of the carbonaceous materials used in the preparation of chars are given in Table I.
Experimental Methods
High-temperature slag–carbon reaction
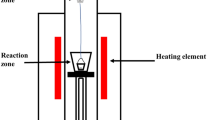
Experiments to study the reduction reaction of FeO in the slag by solid char were performed in the DTF which is schematically shown in Figure 2. The DTF is constructed using an electric resistance heating high-temperature vertical tube furnace (VTF) with a recrystallized alumina tube (VTF-1700/50, internal diameter 88 mm × length 1000 mm) and an isothermal reaction zone (± 5 °C) of 250 mm. The furnace is coupled with a Hiden HPR 20 Quadrupole Mass Spectrometer (QMS) to monitor gaseous products evolving from the reaction. To protect the QMS from soot and condensable tar, a disposable inline filter (Parker 1/4in G nylon) with maximum flow rate and working temperature of 152 L/min and 110 °C, respectively, was installed along the connection line. The DTF-QMS combination allowed the slag samples containing FeO to be heated to pre-set reaction temperatures of 1450 °C, 1475 °C, 1500 °C, and 1525 °C and then chars particles were injected into the molten slag.
Approximately 20 g of slag samples were placed in an alumina crucible (height 65 mm × diameter 42 mm) on the alumina pedestal and lifted to the hot zone of the DTF. The char particles were injected into the molten slag in the pre-set temperature zone through a particle feeder which uses a tee piece connected to two ball valves and an argon line to create an inert atmosphere and carry the particles to the reaction zone during injection as shown in Figure 2. The particle feeder was mounted to the top water-cooled flange and directly connected to an alumina lance (internal diameter 5 mm) inserted through the flange into the top of the crucible containing the slag in the DTF hot zone. Considering the ash content of each material is different and assuming the volatile matters are completely liberated, the carbon element with FeO is calculated to be 1:1 within the char weight to fully reduce iron oxide to metallic iron and if the reaction products are Fe and CO only. Before the experiment starts, a char sample of approximately 225.5, 204.2, and 226.9 mg produced from TC, CC, and BGC, respectively, was placed on the seat of the bottom ball valve on the “off” position, and Ar (with a 99.999 pct purity) flushed through the feeder to create an oxygen-free atmosphere and then closed all the valves. While the furnace was heated to the desired temperature at the heating rate of 10 °C/min, a carrier gas (Ar, 99.999 pct purity) at the flow rate of 1 L/min was purged from the furnace bottom to ensure an inert atmosphere.
When the furnace temperature reached the pre-determined high temperature, the alumina lance was lowered into the crucible directly above the slag. The valve holding the char sample was then opened, and at the same time, the valve controlling the Ar gas was switched to the feeder for ~ 10 seconds to maintain the atmosphere while the char samples were injected into the molten slag in the furnace. The experiment was continued for 1 hour, and during that time, the QMS was set to measure readings of the following gaseous products: N2, O2, CO, CO2, Ar, H2O, H2, CH4, and C2H6. Then the furnace was cooled down at the rate of 5 °C/min to room temperature in an Ar atmosphere, and the samples were collected for further analysis.
Wavelength-dispersive X-ray fluorescence (WDXRF)
The wavelength-dispersive X-ray fluorescence (Rigaku Primus IV WDXRF with a Fluxana RAW package) was used for chemical analysis of the reduced slags to determine the total iron content to measure the extent of reduction. Prior to commencing the sample tests, the machine was calibrated against standards fused beads to produce accurate results. The samples were prepared into 40-mm fused beads using fully calibrated Fluxana Vitriox Electric fuser. The powders were fully dried prior to weighing (1 g sample to 8 g flux). These prepared sample beads were placed in the XRF machine, and then the XRF chamber was evacuated, and the sample was ionized with X-rays, which allows the iron content to be measured accurately in a cross section.
Results and Discussion
Effect of Temperature
To understand the effect of temperature on the reduction rate of FeO in molten slag and gas evolution, the DTF experiments for TC, CC, and BGC char injections were conducted at four temperatures of 1450 °C, 1475 °C, 1500 °C, and 1525 °C, respectively. It was observed that the rate of FeO reduction increases with increasing the reaction temperature and the ratio of gas composition measured changes. As an example, Figure 3 shows the rate of CO and CO2 gas evolution with the time during the reduction of FeO in the molten slag by injection of solid chars produced from CC at four temperatures. Note that the rates of gas formation are in mL/s with no surface area included. This is because there is no fixed contact area, and the particle sizes are expected to change (swell or shrink) during the reaction therefore the contact surface was unknown. There was a short incubation period (15 to 20 seconds) at the beginning of each reaction due to slow reaction at that time and the time required for gases to travel from the reaction zone to the detection point of the online QMS, which is similar to the findings reported by Bafghi et al.[15] and Sun and Easman.[34] The traveling distance and gas mixing within the furnace may cause consistent slight delay in the gas detection; as a result, there is a small amount of gas measured for a period of time after the process is completed.
The char particles injected were devolatilized and to confirm the gas species generated are from slag–carbon reaction only, the QMS was set to analyze CO, CO2, H2O, H2, CH4, and C2H6 gases. These gases are the main gas species produced during thermal treatment of carbon materials (determined through a calibration run where m/z (the mass-to-charge ratio) 0–50 scanned).[35] The main gas species detected were CO and CO2 in the Ar balance, while the content of the other gas species was insignificant.
Some common phenomena can be observed for the reaction at all the temperatures, as shown in Figure 3. The gaseous product from all the tests consists of both CO and CO2, and there are two peaks for CO with one sharp peak which requires different time length to reach its maximum value depending on the reaction temperature. The second peak is partially superimposed on the late phase of the first peak, and it could be from the bubble explosion. This behavior is more obvious at lower reaction temperature due to slower reaction rate, and it suggests that the mechanism for reduction reaction may change during the test. When the char particles are first injected, the FeO concentration in slag is high; therefore, the reduction rate is controlled by Boudouard reaction. After a short period of reaction, FeO concentration is decreased and Boudouard reaction is weakened. Therefore, the reaction controlling mechanism shifts from the chemical reaction to liquid-phase mass transfer or mixed-controlling mechanism which agrees with the other researchers’ findings on similar reactions.[11,12,15] As shown in Figure 3. the rate curve for CO2 appears to be approaching zero after ~ 500 seconds for all temperatures, this means the reaction slows down beyond that point and the gas–slag reaction (Eq. [2]) is slower than the gas–carbon reaction (Eq. [3]) which again confirms the shift in the controlling step. Moreover, the time required for the first CO peak to reach the maximum value decreases with an increase in the reaction temperature, approximately 190 seconds for 1450 °C, 170 seconds for 1475 °C, and ~ 100 seconds for both 1500 °C and 1525 °C.
Figure 4 shows the volume of gas species generated from the slag–char reactions at different temperatures. The results in Figure 4 show similarity in the behavior of all three carbonaceous materials, i.e., in general, the gas volume increases with an increase in the reaction temperature. TC produced a larger amount of CO2 at all temperatures compared to the two biomass materials (BGC and CC). This behavior indicates that the reduction rate is controlled by the Boudouard reaction, since carbon gasification is responsible for gas variation. Gas diffusion in the halo can also be considered to have an influence on the rate-controlling step at the first stage of the reaction. In the authors’ previous findings,[36] TC was shown to be less reactive with CO2 while CC was the most reactive material. From this, it can be assumed that the chemical reaction at the gas–carbon interface (Eq. [3]) is the rate-limiting step for the first stage of the reaction. Also, these results confirm that the gas variation and reaction paths are critically dependent on carbon type used as a reductant.
Extent of FeO Reduction in the Molten Slag by Different Chars
The degree of FeO reduction in the molten slag by char particles produced from the three carbonaceous materials was determined at the temperatures ranging from 1450 °C to 1525 °C. The extent and the rate of the reduction were determined from CO and CO2 gas measurements recorded by QMS during the reactions. The amount of oxygen removed, which equates to the moles of FeO reduced, is calculated from CO and CO2 gas measurement. By knowing the initial weight of the FeO in the slag, the amount of FeO remaining in the bulk slag can be obtained with time by the QMS measurement. To confirm the results obtained via QMS, the reduced slag sample at 1500 °C was examined using WDXRF for quantitative analysis. The WDXRF results have shown a consistent increase of Al2O3 in slag during all experiments. The reactions observed for different chars at temperatures ranging from 1450 °C to 1525 °C are presented in Figure 5, where decrease in the FeO content of the slag with reaction time is shown. To present correct concentration for remaining FeO in the reacted slag, the increase in the Al2O3 was carefully taken into consideration. The extent and rate of FeO reduction in slag vary with the chars made of different carbonaceous materials, suggesting that the reaction kinetics are influenced by chemical and physical properties of solid chars. The reduction process proceeds through two steps, i.e., an initial fast reduction period and a slow secondary stage when the FeO concentration in slag is low. Clearly, the initial reduction rate of FeO by CC chars is the fastest, followed by TC chars almost for all temperatures.
However, the final concentration of FeO in the slag was similar for all three materials at 1450 °C and 1475 °C. The TC char particles appear to have higher reaction rate at initial stage compared to BGC chars but the final concentration of FeO in the slag was similar after 1 hour at 1500 °C as shown in Figure 5(c). The extent of FeO reduction achieved in the first 500 seconds under same conditions is over 60 pct for CC char, ~ 50 pct for TC char, and just over 40 pct for BGC char. The extent of FeO reduction with char particles at four temperatures is presented in Table II.
The QMS results in Table II show that the slag reduction degree with CC char was the highest at all temperatures and it has become more significant at 1500 °C and 1525 °C. TC and BGC chars are shown to have similar reducibility with TC char performing slightly better; however, at 1525 °C, the gap was widened, and TC char performed much better (82.19 pct reduction degree) compared to BGC char (77.75 pct reduction degree). On the other hand, WDXRF analysis on the reacted slags (Table II) confirmed that the extent of reduction was the highest for reaction with CC char at 1500 °C, while TC and BGC chars achieved similar reduction degree. The extent of reduction determined by QMS gas analysis was slightly greater than those measured by WDXRF for all reactions at 1500 °C. Overall, the reduction degree by different char particles calculated by QMS gas analysis is in good agreement with that determined by WDXRF slag analysis.
With regard to the obvious difference in the reduction degree by different char particles in the initial stage (the first 500 seconds in Figure 5), this may be attributed to the difference in their physical and chemical properties of different char particles such as crystalline structure, particle surface roughness, and ash content, which ultimately control the slag interaction with solid carbon particle and governing carbon reactivity.[36] Biomass samples (CC and BGC) have non-compact, low dense fibrous structure, and capillaries from the parent materials are still present, while TC char particles are very compact with more spherical shape and smooth and non-porous surface.[36] The structure and capillaries on biomass char surface contribute to the relatively larger specific surface for interaction. The authors’ previous study for TC and CC confirms that CC contains disordered graphite structure and is more amorphous compared to TC which will enhance the reactivity of CC.[35] The study by Huang et al.[16] proposes that the FeO reduction by solid carbon is controlled by wetting characteristics of the carbonaceous materials and the carbon crystalline structure governs the interaction with the slag, while the same study suggests that carbonaceous material ash content does not influence the slag–carbon interaction. In contrast, many other researchers[9,14,28,30,37] suggested that the ash content has strong influence on the reaction rate between slag and carbon. Assuming the chemical reactions (at char–gas and slag–gas interfaces) control the reduction process, then direct contact is not required, but gas diffusion between carbon and slag is necessary. The minerals (oxides) in carbonaceous materials can be partially or fully fused to form a physical layer on the carbon surface which can act as a physical barrier to inhibit or even prevent CO and CO2 penetration in the halo. The ash content of the carbonaceous materials selected is 8.8, 1.8, and 9.6 pct for TC, CC, and BGC, which shows that there is a correlation between ash content and FeO reduction rate for different char particles. Hence, it can be concluded that an increase in the ash content has a negative effect on the reducibility of carbonaceous materials.
Isothermal Kinetics Analysis
An isothermal kinetics study was carried out in this work on the rate and mechanism of iron oxides reduction by solid carbon. The reduction degree (α) determined from QMS gas analysis allows the model-fitting methods to be adopted. Previous researchers[38,39] used the first-order model for the FeO reduction by solid carbon in the molten slag; however, this study examined different models available and find the most suitable model which can describe the reaction kinetics.
Kinetic Models of FeO Containing Slag Reduction
The reduction degree (α) of iron oxides in the slag is defined as the ratio of the oxygen removed by solid carbon at a given time t to the initial oxygen (i.e., the total oxygen of FeO in the starting slag), which can be expressed by Eq. [4].[40] Please note the removed oxygen by reaction with solid carbons is determined through off-gas measurements for CO and CO2 recorded by QMS. These results are subsequently used to calculate the real-time conversion of FeO in the slag system to allow the model-fitting method to be used for the kinetic analysis.
where \(mi\) is the initial weight of the total oxygen in FeO, \(mt\) is the instant weight of the total oxygen in FeO at time t, which is calculated from the amount of oxygen removed at the time, forming CO and CO2 gases measured by QMS during the reactions.
The reaction rate for FeO reduction being function of (α) can be described by Eq. [5].
where α, \(\frac{{\text{d}}\alpha }{{\text{d}}t}\), k(T), and f(α) denote the reduction degree, reduction rate, temperature-dependent rate constant, and reaction function, respectively.
The reaction rate constant k (T) can be expressed by the Arrhenius Eq. [6]:
By combining Eqs. [5] and [6], the overall reaction conversion rate can be expressed by Eq. [7]:
An integral form of Eq. [7] is written as Eq. [8].[41,42]
where \(G\left(\alpha \right)\) is the integral form of reaction models shown in Table III, while T, R, t, A, and Ea are the absolute temperature (K), ideal gas constant (J/mol/K), the time (s), pre-exponential factor (s−1), and activation energy (kJ/mol), respectively.
Conversion Degree (α)
The isothermal reduction of FeO in the slag by chars from the three carbonaceous materials was conducted at the temperature range of 1450 °C to 1525 °C. The reduction degree of FeO is obtained for each reductant through measurement of evolved gases from their reactions with the slag using Eq. [4]. As an example, Figure 6(b) shows the FeO reduction degree by CC chars as a function of time at four temperatures. It can be seen from Figure 6 and Table II that the reduction rate and conversion degree of FeO increased sharply with increasing temperature. There is a linear increase in the conversion degree at the initial stage of reduction for all temperatures, which then plateaued after a certain reaction time. The temperature increase resulted in the earlier completion of the initial stage and earlier occurrence of the plateau. When the temperature increased from 1450 °C to 1525 °C, the completion time for the initial stage reduced from ~ 300 to 270 seconds for the reaction with CC char, also the conversion degree at the initial stage increased from ~ 35 pct to over 50 pct (Figure 6(b)).
Reaction Mechanism
As previously stated, the reduction of FeO in slag by solid carbon may be controlled by chemical reactions at carbon–gas or slag–gas interface, mass transfer in the slag to interface, and/or diffusion through liquid or gas halo formed around solid carbon particle. The kinetic models which can describe these reaction mechanisms to determine the reaction rate are categorized into random nucleation and subsequent growth (A), diffusion (D), geometric contraction (R), and chemical reaction-order models (F).[44,46,47] Dickinson and Heal[43] reported 27 kinetic models in total and those well-established models are listed in Table III.
ln–ln analysis method
The Avrami–Erofeev equation is widely used to analyze isothermal kinetics;[47,48] therefore, the model is employed to describe the reduction kinetics of slag containing FeO in this study. The Avrami–Erofeev equation can be written as below[48]:
By taking the logarithm of both sides of Eqs. [9], [10] is derived:
where \(a\) is the conversion degree, k is the reaction rate constant, t is the time, and n is Avrami exponent, which provides information about the conversion mechanism involved during the reduction process [48]. The n values for all the selected models are presented in Table III.
To evaluate experimental data and select the suitable model, ln [− ln (1 − a)] is plotted against lnt using Eq. [10]. The slope and intercept of the fitting lines are n and nlnk, respectively. Figure 7 shows the experimental data fitted into Eq. [10] for CC char reaction, and the slop n and intercept nlnk for reactions with different reductants are presented in Table IV. Regardless of the reductant used, the first stage values of the slope n were between 0.79 and 0.90 for the temperature range studied. These values are closely matching with n value for F2 model 0.83 in Table III. Therefore, it can be speculated that the rate-limiting step is chemical reaction for the first stage of the reaction, then the second-order model F2 is proposed and represented by the kinetic equation: (1 − α)−1 − 1 = kt.
On the other hand, the slope n vales for second stage are between 0.38 and 0.57, and considering most of the values are less than or closely matching with 0.54, then three-dimensional diffusion is the proposed model and represented by the kinetic equation: [1 − (1 − a)1/3]2 = kt. From these results, it can be stated that gas diffusion or mass transfer of FeO in the liquid phase to the slag–carbon interface is the rate-limiting step when FeO concentration decreased in the second stage of the reaction.
Moreover, the reaction with CC char resulted in much higher conversion in the first stage of the reaction followed by TC and BGC chars. This phenomenon is due to the higher reactivity of CC chars compared to the other two selected carbons.[36] This indicates that the carbon type can have a great influence on the rate of reaction, and chemical reaction is the controlling mechanism for the first stage. Regardless of the carbon type used, the conversion degree is more similar at a given temperature for reactions at the second stage. This shows the influence of the carbon type on the rate is weakened and the controlling mechanism shifts at the second stage.
Model-fitting method
Figure 8 shows the linear fitting of G(α) against time (t) for the selected model functions for the two reduction stages using Eq. [8]. The slope represents the reaction rates k(T) at a given temperature for reaction with chars from different carbon sources. The values of k(T) obtained at different reduction stages and the details of the mechanism which is likely to control the reaction steps are represented in Table V.
Based on the data fitting for ln − ln analysis method for the first stage, the best fitting function was confirmed as (1 − α)−1 − 1 for all tests, which represents a second-order model. As shown in Table V, the correlation coefficients (R2) values for the first stage of the reactions are between 0.98 and 1.00, which confirms that the regression model fits very well with the observed data. Regardless of the materials used as a reductant, the value of k(T) increased with the temperature increase. CC char as a reductant has the highest k(T) values which is related to the material’s higher reactivity in the first stage while the values for TC and BGC chars were much closer.
As for the second stage, the best fitting function is [1 − (1 − a)1/3]2, which describes three-dimensional diffusion model. The correlation coefficient (R2) values for the second stage of the reactions are between 0.93 and 1.00, and the correlation is good for most of the linear graph in Table V. Again, the reaction rate k(T) with CC chars is the highest followed by TC and the BGC chars; this shows that higher reactivity of CC char also influences the second stage of the reaction.
Reaction Activation Energy
The activation energy Eα values for the two reaction stages can be obtained based on the Arrhenius equation. By taking logarithm of Eq. [6], the Arrhenius equation is written in the below form[47]:
Figure 9 shows the linear fitting of lnk(T) vs 1/T for different char reaction at different reduction stages using Eq. [11] to calculate Eα. The CC char has the lowest Eα of 229 kJ/mol for the first stage of the reaction, while TC and BGC chars have higher Eα with 290 kJ/mol and 267 kJ/mol, respectively. These Eα values correlate reasonably well with other findings in the literature that the range of 41 to 97 kcal/mol (171.5 to 405.8 kJ/mol) has been reported for reaction between slag with similar properties and solid carbon from coal char or graphite.[12,21] Min et al.[12] have predicted the chemical reaction at the carbon surface likely to be the rate-limiting step with measured Eα of 60 kcal/mol (251 kJ/mol) for the reaction of the slag containing 10 pct FeO with graphite at similar temperature range. The values obtained in the present study are in the good agreement with the results obtained by Min et al.[12] therefore, chemical reaction at carbon interface is likely to be rate controlling for the first stage of the reduction reaction.
In contrast, reaction with CC char resulted in the highest Eα (369 kJ/mol) for the second stage which is much higher than the first stage for the same material. The Eα values for both TC and BGC char reactions were (265 and 282 kJ/mol, respectively) for the second stage which are much closer to their first stages and smaller than the Eα obtained for CC char. As shown in Table IV, the conversion degree for the first stage reaction with CC char was the highest for all temperatures, while TC char was the second highest. This higher conversion leads to much lower FeO concentration when the second stage begins for CC reductant compared to the second stage for TC and BGC chars. It appears that the lower FeO concentration resulted in higher Eα value for the second stage for CC, due to lower gas formation which results in lower agitation of the slag layer. As for the second-stage reaction with TC char, the Eα value was lower than the first stage despite achieving the second best conversion for the first stage. This indicates that the liquid-phase mass transfer influences the reaction mechanism for the second stage of the reaction. On the other hand, despite achieving the lowest conversion (α) in the first stage as shown in Table V, the second stage of the reaction with BGC was slow which indicates that the chemical reactions at char interface is still likely to influence the controlling mechanism. Therefore, it can be speculated that this slower reaction behavior for BGC in the second stage is due to higher ash content. As the carbon particle shrinks due to conversion on the surface, the ash content increases and it covers the surface of the carbon particle to act as a physical barrier to inhibit or even prevent CO and CO2 penetration in the halo. Considering these concerning elements, the second-stage reaction is likely to be controlled by more than one mechanism, and the mechanism controlling the reaction is expected to be the mixed limiting step.
Conclusion
The focus of this study has been on the reduction of FeO in the synthetic HIsarna slag (CaO–SiO2–Al2O3–MgO–FeO) using chars from three carbon sources. The reduction rate of FeO in the slag was measured using the mass spectrometer and confirmed with slag analysis, and the kinetic parameters are evaluated from these results. The following conclusions can be drawn from this study:
-
A strong impact of temperature on the reduction rate of FeO in slag by chars is found that the reduction rate increases with the increase in temperature. This is also demonstrated by the significant effect of temperature on the variation of gas products CO and CO2.
-
The FeO reduction process can be divided into two steps: the first stage with a sharp increase in the conversion degree with time and a slow second step. In the first 500 seconds, the reduction degree achieved at 1500 °C was over 60 pct by CC char, ~ 50 pct by TC char and just over 40 pct for BGC char. Furthermore, the slag reaction with CC char resulted in the highest final degree of reduction under all temperatures, but the difference between TC and BGC char was smaller with TC performed slightly better at higher temperatures.
-
The first stage of the reaction is represented by the second-order model (F2), and the activation energy values obtained for the reactions with TC, CC, and BGC chars were 290, 229 and 267 kJ/mol, respectively. The first stage of reaction rate is controlled by chemical reaction at the solid carbon interface, while for the second stage, described by three-dimensional diffusion model (D3), mixed influence from gas diffusion, liquid-phase mass transfer, chemical reaction, and carbon diffusion are likely to control the reduction rate.
References
K. Meijer, C. Zeilstra, C. Teerhuis, M. Ouwehand, and J. Van Der Stel: Trans. Indian Inst. Met., 2013, vol. 66, pp. 475–81.
A. Hasanbeigi, M. Arens, and L. Price: Renew. Sustain. Energy Rev., 2014, vol. 33, pp. 645–58.
Y. Junjie: Int. J. Miner. Process., 2018, vol. 3, pp. 15–22.
Y. Qu, Y. Yang, Z. Zou, C. Zeilstra, K. Meijer, and R. Boom: Ironmak. Steelmak., 2015, vol. 42, pp. 763–73.
Y. Qu, Y. Yang, Z. Zou, C. Zeilstra, K. Meijer, and R. Boom: ISIJ Int., 2015, vol. 55, pp. 952–60.
M. Abdul Quader, S. Ahmed, S.Z. Dawal, and Y. Nukman: Renew. Sustain. Energy Rev., 2016, vol. 55, pp. 537–49.
Z. Chen, C. Zeilstra, J. van der Stel, J. Sietsma, and Y. Yang: Ironmak. Steelmak., 2019, vol. 1, pp. 1–7.
Y. Qu, Y. Yang, Z. Zou, C. Zeilstra, K. Meijer, and R. Boom: ISIJ Int., 2014, vol. 54, pp. 2196–205.
J.W.K. Van Boggelen, H.K.A. Meijer, C. Zeilstra, H. Hage, and P. Broersen: in SteelVIA, 2018, pp. 1–7.
B. Sarma, A.W. Cramb, and R.J. Fruehan: Metall. Mater. Trans. B, 1996, vol. 27B, pp. 717–30.
S. Kwangyong and R.J. Fruehan: ISIJ Int., 2000, vol. 40, pp. 7–15.
D.J. Min, J.W. Han, and W.S. Chung: Metall. Mater. Trans. B, 1999, vol. 30B, pp. 215–21.
S.R. Story, B. Sarma, R.J. Fruehan, A.W. Cramb, and G.R. Belton: Metall. Mater. Trans. B, 1998, vol. 29B, pp. 929–32.
M.S. Bafghi, H. Kurimoto, and M. San: ISIJ Int., 1992, vol. 32, pp. 1084–90.
M.S. Bafghi, H. Kurimoto, and M. Sano: ISIJ Int., 1992, vol. 32, pp. 280–86.
X.A. Huang, K.W. Ng, L. Giroux, and M. Duchesne: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 1387–98.
F. Fun: Metall. Trans., 1970, vol. 1, pp. 2537–41.
S.L. Teasdale and P.C. Hayes: ISIJ Int., 2005, vol. 45, pp. 642–50.
M. Ozawa, S. Kitagawa, S. Nakayama, and Y. Takesono: Trans. Iron Steel Inst. Jpn., 1986, vol. 26, pp. 621–28.
R.J. Fruehan: Metall. Mater. Trans. B, 1977, vol. 8B, pp. 279–86.
A. Sato, G. Aragane, K. Kamihira, and S. Yoshimatsu: Trans. Iron Steel Inst. Jpn., 1987, vol. 27, pp. 789–96.
F.-Z. Ji, M. Barati, K. Coley, and G.A. Irons: Can. Metall. Q., 2012, vol. 44, pp. 85–94.
F. Ji, M. Barati, K. Coley, and G.A. Irons: 7th Int. Conf. Molten Slags, Fluxes Salts, 2004, vol. Jonhannesb, pp. 399–406.
F. Ji, M. Barati, K.S. Coley, and G.A. Irons: Publ. 60th EAF Conf. Proc., 2002, pp. 511–24.
P. Wei, M. Sano, M. Hirasawa, and K. Mori: Trans. Iron Steel Inst. Jpn., 1988, vol. 28, pp. 637–44.
R.H. Smith and R.J. Fruehan: Steel Res., 1999, vol. 70, pp. 283–95.
D.J. Min and R.J. Fruehan: Metall. Mater. Trans. B, 1992, vol. 23B, pp. 29–37.
J. Biswas, K. Gu, and K.S. Coley: Metall. Mater. Trans. B, 2021, vol. 52B, pp. 3888–3906.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B, 2017, vol. 48B, pp. 2984–3001.
C.L. Molloseau and R.J. Fruehan: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 335–44.
M. Barati and K.S. Coley: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 61–69.
M. Barati and K.S. Coley: Metall. Mater. Trans. B, 2004, vol. 36B, pp. 169–78.
M. Barati, E. Chen, and K. Coley: 7th Int. Conf. Molten Slags, Fluxes Salts, 2004, pp. 393–98.
H. Sun and W. Easman: Energy Fuels, 2007, vol. 21, pp. 413–18.
D. Khasraw, S. Spooner, H. Hage, K. Meijer, and Z. Li: Fuel, 2021, vol. 292, pp. 1–11.
D. Khasraw, T. Theint, X. Yang, V. Degirmenci, H. Hage, and K. Meijer: Fuel, 2021, vol. 309, pp. 1–12.
J.R. Dankwah, P. Koshy, P. O’Kane, and V. Sahajwalla: Steel Res. Int., 2012, vol. 83, pp. 766–74.
R.K. Paramguru, H.S. Ray, P. Basu, and A. Jouhari: Ironmak. Steelmak., 1996, vol. 23, pp. 328–34.
P. Basu and H.S. Ray: J. Therm. Anal., 1995, vol. 45, pp. 1533–40.
Y.S. Sun, Y.X. Han, P. Gao, and G.F. Li: Ironmak. Steelmak., 2014, vol. 41, pp. 763–68.
L. Zhang, Y. Zhu, W. Yin, B. Guo, F. Rao, and J. Ku: ACS Omega, 2020, vol. 5, pp. 8605–12.
Y. Zhang, J. Zhao, X. Ma, M. Li, Y. Lv, and X. Gao: Miner. Eng., 2019, vol. 71, pp. 825–37.
C.F. Dickinson and G.R. Heal: Thermochim. Acta, 2009, vol. 494, pp. 1–14.
Y.D. Wang, X.N. Hua, C.C. Zhao, T.T. Fu, W. Li, and W. Wang: Int. J. Hydrogen Energy, 2017, vol. 42, pp. 5667–75.
A. Khawam and D.R. Flanagan: J. Phys. Chem. B, 2006, vol. 110, pp. 17315–28.
Y. Man, J.X. Feng, F.J. Li, Q. Ge, Y.M. Chen, and J.Z. Zhou: Powder Technol., 2014, vol. 256, pp. 361–66.
W. Lv, X. Lv, Y. Zhang, S. Li, K. Tang, and B. Song: Powder Technol., 2017, vol. 320, pp. 239–48.
C. Li, X. Lv, J. Chen, X. Liu, and C. Bai: Int. J. Refract. Met. Hard Mater., 2015, vol. 52, pp. 165–70.
Acknowledgments
DK would like to thank Tata Steel Nederland Technology BV for providing the PhD scholarship (Reference No: COL1421/GIPS03241) to carry out this research. ZL would like to appreciate the funding from EPSRC under the Grant Number EP/N011368/1. The authors appreciate Tata Steel HIsarna team for fruitful discussions and providing samples.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khasraw, D., Yan, Z., Hage, J.L.T. et al. Reduction of FeO in Molten Slag by Solid Carbonaceous Materials for HIsarna Alternative Ironmaking Process. Metall Mater Trans B 53, 3246–3261 (2022). https://doi.org/10.1007/s11663-022-02603-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02603-5