Abstract
Does age substantially affect the emergence of human immune-mediated arthritis? Children do not usually develop immune-mediated articular inflammation during their first year of life. In patients with juvenile idiopathic arthritis, this apparent ‘immune privilege’ disintegrates, and chronic inflammation is associated with variable autoantibody signatures and patterns of disease that resemble adult arthritis phenotypes. Numerous mechanisms might be involved in this shift, including genetic and epigenetic predisposing factors, maturation of the immune system with a progressive modulation of putative tolerogenic controls, parallel development of microbial dysbiosis, accumulation of a pro-inflammatory burden driven by environmental exposures (the exposome) and comorbidity-related drivers. By exploring these mechanisms, we expand the discussion of three (not mutually exclusive) hypotheses on how these factors can contribute to the differences and similarities between the loss of immune tolerance in children and the development of established immune-mediated arthritis in adults. These three hypotheses relate to a critical window in genetics and epigenetics, immune maturation, and the accumulation of burden. The varied manifestation of the underlying mechanisms among individuals is only beginning to be clarified, but the establishment of a framework can facilitate the development of an integrated understanding of the pathogenesis of arthritis across all ages.
Key points
-
The arthritis-free ‘immune privilege’ of early childhood is overridden by multiple mechanisms, progressively and age-dependently, generating recognizable patterns of chronic inflammatory arthritis.
-
The emergence of arthritis involves interconnected mechanisms related to immune priming, to a situational susceptibility and to the accumulation of an inflammatory burden.
-
The accumulation of epigenetic drift may contribute to differences across ages.
-
The exposome is expected to contribute to arthritis emergence in adults as well as in children.
Similar content being viewed by others
Introduction
The timing of the emergence of chronic inflammatory disorders is one of the unsolved conundrums in medicine. Why do some disease phenotypes present during childhood, and others only later in life? A vivid and timely example of how age matters to the development and phenotype of disease is the current COVID-19 pandemic. As the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus spread around the world, it quickly became clear that severe clinical manifestations of COVID-19 were rare in children1. At the same time, a small proportion of SARS-CoV-2-infected children, often with no (or only mild) symptoms in the initial course of the infection, developed a serious and threatening post-infectious hyperinflammatory syndrome known as multisystem inflammatory syndrome in children (also known as paediatric inflammatory multisystem syndrome), which was characterized by fever, skin rash, myocarditis and features reminiscent of Kawasaki disease2,3. A similar syndrome is also seen in adults (multisystem inflammatory syndrome in adults), mainly in young adults. For COVID-19, the most important determinant of disease severity and mortality in adults is age. Clearly, both the initial and late immune responses to SARS-CoV-2 infection and the ensuing clinical phenotypes are age dependent.
This example is not unique. Other age associations are well known, for example in reactive arthritis and polymyalgia rheumatica (PMR). Reactive arthritis tends to occur most commonly in men within the age range 20–40 years and PMR typically affects individuals >50 years old and is responsible for rhizomelic muscle and joint pain. PMR results from unknown triggers4, and its disease pathogeny is thought to be driven by both activated innate immune cells (especially monocytes and macrophages) as potential primary sources of IL-6, and a deregulated adaptive immune compartment (with reduction of numbers of regulatory T (Treg) cells and a shift towards T helper 17 (TH17) and TH1 cells5. In addition, the T cell compartment exhibits a higher frequency of senescence markers (CD28 expression loss) alongside the expression of activating receptor NKG2D6. Understanding how age-related immune-system modifications contribute to PMR occurrence is an unmet need.
A first comprehensive chronological map of human health has been provided recently7. On the basis of approximately four million electronic health records of patients in England, from 1 year old to advanced age, cumulative incidence and period prevalence were estimated for 308 common morbidities. In addition to variation related to sex and ethnicity, the data identified age-related differences throughout the decades of life. Over the lifespan, a shift from atopic disorders and acute infections towards cancers and degenerative diseases at advanced ages was seen. From a musculoskeletal point of view, young children (<10 years old) mostly suffered from wrist fractures, enthesopathy and scoliosis, which between them represented about 4% of morbidities in cumulative incidence, compared with >8% for dermatitis, which was the most common condition in this age range. As age increased beyond the 20–29-year-old stratum, the burden of metabolic and degenerative osteoarticular diseases (including osteoarthritis, osteoporosis and gout) progressively increased, reaching an approximate cumulative incidence of 20% in the 70–79-year-old stratum. These data also confirmed that the median age at first diagnosis for two common immune-mediated arthritides in adults, namely rheumatoid arthritis (RA) and psoriatic arthritis (PsA), peaks during the fifth and sixth decades, respectively. By contrast, most juvenile idiopathic arthritis (JIA) subtypes peak in the first decade of life (between 1 year old and 6 years old)8.
The associations between age and immune-mediated disease are not yet well studied, mainly because of the separation between adult and paediatric patient care and related research programmes. For example, the field of paediatric rheumatology is largely separated from that of ‘adult’ rheumatology. Age-dependent manifestations of inflammatory arthritis have led to separate disease classifications for paediatric rheumatological diseases that have only recently become the focus of a healthy debate9. JIA, which is classified as chronic inflammation involving one or more joints and initiating before the age of 16 years, is only rarely seen before the age of 1 year, and its subtypes differ in their age distributions10,11. RA, both seronegative and seropositive, has specific age distributions over the adult lifespan12, and the course of PsA is also modulated by age. Indeed, skin disease (psoriasis) occurs mostly prior to arthritis in adults yet conversely after arthritis in the juvenile form of PsA13. Interestingly, recent data showed that during the transition period from paediatric to adult care, the proportion of patients fulfilling adult immune-arthritis classification criteria (the ACR–EULAR 2010 criteria for RA, the Yamaguchi criteria for adult-onset Still’s disease, the Assessment of SpondyloArthritis international Society criteria for spondyloarthritis and the Classification Criteria for Psoriatic Arthritis) differ substantially among different subtypes of childhood arthritis, at least at the classification level14. Increased understanding of underlying mechanisms and pathways of immune-mediated arthritis has re-opened the discussion of the relevance of the different classification systems and, along with the development of novel bioinformatics strategies, has created an opportunity to move towards classification with a more biological and molecular basis, as reviewed previously15.
Clearly, age matters in the development of human arthropathies. In this Review, we explore the underlying mechanisms of age-specific arthropathy manifestations and, most importantly, what we can learn from differences and similarities across the age spectrum. We focus on JIA (excluding the predominantly autoinflammatory subtype systemic JIA) and on the most prevalent adult counterparts, namely RA and PsA. We develop three hypotheses (which are summarized in Fig. 1) on how age-related mechanisms trigger chronic arthritis across ages. These hypotheses are probably not mutually exclusive, and likely represent processes that act synergistically and interact with shared molecular mechanisms. By understanding the key immune mechanisms that drive arthropathies in children and adults, we will be able to explore new avenues of diagnostics, treatment and, ultimately, maybe even prevention.
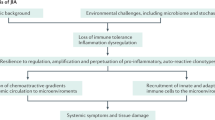
Emergence of arthritis is a multifactorial phenomenon. Given the striking differences observed between paediatric-onset and adult-onset arthritis, age-specific mechanisms are likely to be present. How these mechanisms articulate in individuals is still only beginning to be clarified. We propose an age-related model of arthritis emergence, based on three hypotheses that are not mutually exclusive, and that are potentially even interconnected. The ‘critical window’ hypothesis relates to pro-arthritogenic factors that might define a set point for arthritis emergence. As such, genetic predisposition, epigenetic status and associated comorbidities represent relevant components for this critical window. The immune-maturation hypothesis relates to the fact that the first year of life is usually free of arthritis, suggesting an immune ‘privilege’. Currently available data suggest a low propensity at this age to autoimmune inflammatory responses that is related to regulatory controls such as the presence of a naive immune repertoire, with tolerogenic T lymphocytes, naive dendritic cells and a diverse regulatory T (Treg) cell repertoire. Early microbiota composition, built from fetal stages onwards, may also contribute to this ‘tolerogenic’ environment. However, aberrant priming of the immune system, especially resulting from infection with pro-arthritogenic pathogens (and other unknown mechanisms) could also affect the emergence of arthritis. Finally, the accumulation of a pro-inflammatory burden resulting from acquired influences such as adiposity, smoking, microbiome dysbiosis, immunosenescence and inflammageing, and more widely by the exposome, represent additive hits upon regulatory control, leading to breach of tolerance, and inflammation.
A ‘critical window in (epi)genetics’
Although the exact trigger (or triggers) and pathogenic mechanisms that underlie the development of chronic arthritis in humans are still largely unresolved, it is generally thought that chronic immune arthritis, in keeping with other immune-mediated inflammatory diseases (IMIDs), is the result of exposure of a genetically susceptible host to various environmental stimuli. JIA and RA are both complex, polygenic diseases, in which the timing of specific environmental exposures in individuals with specific genetic backgrounds seems to be critical for disease development. We can therefore hypothesize that differences in age at onset might be explained by inherited predisposition for so-called ‘critical windows’ for environmental influence.
Shared genetic components in arthritis
A major determinant of the emergence and severity of IMIDs is an individual’s genetic background16. The most common inflammatory arthropathy in adults is RA, and results from seminal studies have identified an association with HLA class II alleles in this disease17, leading to the definition of the shared epitope (SE)18, and also identifying a role for the PTPN22 locus19. For JIA, associations with specific HLA regions (which include PTPN22 (ref.20), and which differ depending on disease phenotype) were identified >40 years ago21,22,23. PTPN22 is an ethnicity-specific genetic factor, and the PTPN22 R620W-encoding allele is mostly observed in white European and white North American populations24,25. Altogether, currently available results point to a striking overlap in risk loci between JIA and other forms of arthritis20,22, informing ongoing discussions on the transformation of the current classification criteria for JIA15, as reviewed elsewhere15,26. The overlap of genetic susceptibility loci suggests shared mechanisms among immune arthropathies. Notably, this overlap involves immune arthritis specific to childhood as well as IMIDs specific to adulthood26, and the identification of genetic susceptibility for human arthropathies19,27,28,29,30 has translated into therapeutic targeting of IL-6, IL-17, CTLA-4–CD28 and Janus kinases31. However, many genetic associations are still poorly understood, and shared genetic backgrounds are sometimes associated with opposite outcomes, such as the protective nature of HLA-DRB1:04 in polyarticular JIA, in contrast to its status as a risk allele in seropositive adult RA20,22,23,31,32,33. Similarly, HLA-DRB1:08 is a risk allele for polyarticular JIA, whereas it is protective against adult seropositive RA. By contrast, the PTPN22 rs6679677 single-nucleotide polymorphism (SNP) confers risk in both conditions. Novel bioinformatics strategies may help to develop our understanding of these findings and to identify relevant non-coding variants, in particular those that function by modulating the binding of DNA regulatory proteins such as transcription factors, including epigenetic marks and predicted and/or experimentally determined transcription factor binding motifs.
Although the common dogma is to classify paediatric and adult rheumatological IMIDs separately, genetic data from the past decade have challenged this age-dependent partitioning of arthritis, raising the question of why these conditions should be separated rather than being grouped together. Evidence of similarities in genetic backgrounds in child and adult immune arthritis has led to the proposal of a new classification system26. This proposal combines genetic patterns, demographic and clinical data to generate four clusters spanning both paediatric and adult variants of inflammatory arthritis: seropositive, seronegative, spondyloarthritis and systemic. This classification needs further development to decipher intra-cluster heterogeneity and to determine the involvement of particular immune pathways, which could translate to different therapeutic targets. However, this new paradigm is valuable, and an open mind and pragmatic approach might help us to develop the current step-up treatment approach into more tailored strategies15.
Epigenetics and phenotypes
Control of gene expression can be postulated to account for immune arthritis onset and outcome. The discovery of epigenetic regulation — defined as heritable (and acquired) modifications of (regulatory) DNA sequences, affecting the expression of genes without changing the coding sequence itself — has revolutionized our understanding of immune arthritis34,35,36. Epigenetic mechanisms are thought to act at the interface between disease risk factors (including environmental factors, nutrition, infection and socioeconomic factors) and the implementation of the genetic information encoded in DNA37,38,39.
DNA methylation and histone modification are well described epigenetic marks. Current technologies can provide accurate and reproducible genome-wide analysis of DNA methylation patterns40. Notably, methylation seems to induce stable, long-term gene repression41. The deregulation of methylation in RA might account for aggressive fibroblast phenotypes42,43. In murine arthritis, repeated inflammatory insults with zymosan or monosodium urate crystals induce metabolic, transcriptomic and epigenetic changes in synovial fibroblasts. These changes are mediated by complement components C3 and C3a receptor, and result in pathogenic inflammatory fibroblast functionality44. An elegant experimental approach has enabled differentiation of methylation signatures that cause RA from those that result from the disease. In this approach, peripheral blood leukocytes from 354 anti-citrullinated protein antibody (ACPA)-positive patients with RA and 337 healthy individuals from the Swedish Epidemiological Investigation of Rheumatoid Arthritis (EIRA) study were assessed45. Results were adjusted according to blood-cell proportions, age, sex and smoking status. Ultimately, the results of a series of conditional correlation analyses identified causal inference on RA occurrence for a set of 10 differentially methylated positions, 9 of which were located in the MHC cluster, emphasizing the relevance of MHC genes, and hence core immunological function (as opposed to primary non-immunological stromal-cell dysregulation) in RA susceptibility. Results from a landmark study of disease-discordant monozygotic twins support the importance of DNA methylation signatures in the emergence of autoimmune diseases such as RA46.
In JIA, evidence suggests that epigenetic profiles and alterations have important roles in disease development47,48,49. Immunoprecipitation targeting a specific histone modification (acetylation of lysine at residue 27 of histone H3) has demonstrated that synovial fluid-derived CD4+ effector memory T cells display a disease-specific signature of both enhancers and super-enhancers, which are non-coding regulatory elements in cis-acting DNA sequences ranging in size from a few hundred base pairs to some 50 kb, to which transcription factors and co-factors can bind to control transcription48. In systemic lupus erythematosus (SLE), a specific mechanism that contributes to T cell and B cell hyperactivity involves reduction of expression of the transcription factor RFX1, which affects DNA methylation and histone acetylation in CD4+ T cells50. Clinical trials with compounds that affect epigenetic alterations, including enhancer and super-enhancer activity, are ongoing in the field of oncology51, demonstrating that it is possible to target disease-specific signatures of regulatory elements that affect immune-related genes. Important reader proteins at enhancer and super-enhancer regions are the bromodomain and extraterminal (BET) proteins, which can bind to acetylated histone and non-histone proteins, thereby regulating gene transcription. BET inhibitors impair differentiation of naive CD4+ T cells into effector T cell subsets52,53.
Further evidence of the potential for therapeutic modulation of epigenetic markers in T cell-mediated autoimmune diseases is provided by the observation that the DNA methyltransferase inhibitor 5-azacytidine can modify the surface glycoproteins (T4 molecules) expressed upon maturation of thymocytes54. Methotrexate, the cornerstone of arthritis treatment, is also expected to modulate DNA methylation. Methotrexate inhibits folate metabolism and, as such, should reduce regeneration of methionine, which is an essential methyl-group donor for DNA methylation55. Results indicate that the DNA hypomethylation profile that is found in RA and JIA is modified by methotrexate treatment56,57. Furthermore, in a study of patients from the Scottish Early RA cohort and additional samples from an independent cohort, a chromosome conformation signature was found to predict response to methotrexate prior to treatment initiation58. This approach identified at baseline non-responders to a 6-month course of methotrexate, with a true-negative response rate of 86% and 90% sensitivity.
Age-related epigenetic drift
The epigenome is particularly susceptible to deregulation at pivotal and extreme ages, especially during early embryonic, neonatal, pubertal and elderly periods59. In this respect, lessons from Alzheimer disease may be valuable for arthritis. One theory is that Alzheimer disease originates early in life60. Although it is associated with mutations (in genes such as APP, PSEN1 and PSEN2), Alzheimer disease develops with a sporadic non-Mendelian pattern, strongly suggesting a role for epigenetic regulation. The accumulation of epigenetic modification (including histone modulation and DNA methylation) in the susceptible early embryonic and neonatal periods, or later in life, is thought to contribute to Alzheimer disease61. Whether and how non-Mendelian IMIDs, such as the human chronic arthropathies, similarly arise from an individual’s heritable epigenetic drift is not yet understood62. In the differentiation of human Treg cells into effector Treg cells in arthritis, the cells display a transcriptional profile similar to that of naturally occurring (suppressive) Treg cells, along with characteristics of an effector program (including transcription of TNFRSF18, PRDM1 and BATF). This profile suggests that the specific transcriptional and epigenetic signature of Treg cells from the site of inflammation, the synovial fluid and the joint, is developed or shaped through epigenetic changes and environment-specific adaptations47.
Age-related comorbidities
Both in paediatric arthritis and in adult arthritis, specific, strikingly different, comorbidities exist or develop. These comorbidities can provide information about specific disease mechanisms across the age spectrum (Box 1).
Chronic anterior uveitis (CAU) is the most common extra-articular disease manifestation of JIA, and although its prevalence differs between subtypes, it can be seen in as many as 25–30% of anti-nuclear antibody (ANA)-positive patients with oligoarticular JIA63,64. CAU presents completely asymptomatically, so it must be actively screened for, and it is common in all JIA subtypes except for systemic JIA and rheumatoid factor-positive polyarticular JIA. In adults, acute uveitis is usually symptomatic (with pain and red eye) and largely limited to the spondyloarthropathies. Acute uveitis can also develop in adolescents with spondyloarthropathy. Known risk factors for the development of CAU in patients with JIA are JIA subtype (patients with oligoarticular JIA are at highest risk), younger age at disease onset and the presence of ANAs63. Evidence has linked JIA-associated uveitis with specific HLA haplotypes (HLA-DR5 and HLA-DRB*1104)65,66, whereas other HLA haplotypes (HLA-DR1 and HLA-DQA*0101) seem to be protective. Although B cells are generally considered not to have a central role in the pathogenesis of JIA, results from transcriptomic and proteomic analysis of iris tissue and aqueous humour suggest the presence of disease mechanisms involving B cells67. Appreciation of specific disease manifestations or even disease subtypes has resulted in stratified clinical trials in JIA68. Although CAU associated with JIA is different from acute anterior uveitis that is seen in adult spondyloarthropathies, it seems for both comorbidities that adalimumab and other anti-TNF monoclonal antibodies are preferable to etanercept (a TNF-binding receptor fusion protein), which indicates the potential for the development of targeted treatment modalities69.
Apart from uveitis, other symptomatic comorbidities in adults accumulate over time and substantially influence arthritis. Psoriasis is a hallmark of PsA. With a prevalence of 6–42% among patients with psoriasis, PsA is the most frequent comorbid condition that is associated with psoriasis70. In most cases, the skin disease precedes the articular disease. Importantly, pathogenic pathways governing psoriasis and PsA do not fully overlap, and some differences (such as the discrepancies between the clinical responses to DMARDs) may occur as a result of tissue-specific responses71,72. However, leakage of immune deregulation from the skin to the joint is suspected73, potentially involving lymphocyte subsets such as CD8+ T effector memory CD45RA cells74 and CD8+ CCR10+ T cells75. DNA methylation patterns of CD8+ T cells can differentiate patients with psoriasis from those with PsA, suggesting the potential for early patient stratification for therapy76. Another comorbidity in adult chronic arthritis is an increased cardiovascular risk. Psoriasis is also associated with ischaemic heart disease, cerebrovascular and peripheral vascular disease, and with subsequent mortality77,78,79.
Results from multiple studies suggest that psychological disorders influence the immune system and the course of autoimmune diseases. The occurrence of depression is a common comorbidity in adult patients with psoriasis80, and the risk of developing psoriasis is higher in patients suffering from depression than in those without depression, suggesting a bidirectional interaction. The influence of depression on the development of arthritis is exemplified in RA, in which it is the most frequent comorbidity, and is responsible for a dramatic effect on quality of life81,82. In addition, results from population-based studies have shown that depression is associated with elevation of the risk of developing RA, with any protective role of antidepressant medication being unclear83,84. This association is not epidemiologically and pathophysiologically limited to adulthood85,86. Exposure to adverse childhood experiences is associated with the risk of reporting JIA, with an adjusted odds ratio of 9.4 (95% CI 4.0–22.1) for arthritis versus health in individuals with four or more adverse childhood experiences87. Current hypotheses to explain this association note the potential role of stress in the induction of pro-inflammatory cytokines and in insufficient glucocorticoid-related negative feedback on inflammation in adulthood88,89.
The immune-maturation hypothesis
Throughout early life, individuals face immunological challenges that shape the immune system, building on the base of the innate immune system90 and gradually acquiring and refining an adaptive immune repertoire91,92. This acquisition begins in utero and potentially shapes later autoimmune outcomes. The development of chronic arthritis in humans probably involves multiple potential triggers both in the joint and in lymphoid organs.
Age-related features of synovial tissue
Little is known about changes affecting synovium and immune-cell surveillance of the joint during growth and ageing. Normal fetal synovium sampled postpartum (at term) is histologically similar to adult synovium93. In morphological analysis, normal juvenile synovium seems to be similar to adult tissue94. Microarthroscopic and microscopic synovium assessment demonstrate that, compared with adult synovium, that of teenagers (15–19-years old) displays less ‘roughness’ of the whole tissue (fewer villi), with a regular vascular network, a preponderance of elongated and evenly distributed cells, and fewer macrophages. In addition, adipocyte content decreases with age95. These observations may, at least in part, explain features such as the differences in antibody-dependent and immune-complex-mediated involvement in synovitis and arthritis across the age spectrum96,97. From a molecular point of view, the autoantibodies in seropositive RA can result in immune-complex deposits and formations on cartilage, which can subsequently potentiate joint inflammation. This phenomenon might be facilitated in cartilage of older people, which is theoretically more susceptible to complement-binding through loss of sialic acids in the surface section97,98,99. To date, no comprehensive comparison of the composition and specific phenotype of paediatric and adult synovial cells exists.
Maternal influences
The maternal imprint may affect the immune system of the child and bias its propensity to trigger immune arthritis (Fig. 2). This phenomenon is apparent at various levels, such as in the vertical transmission of immunity to specific pathogens (by the transplacental passage of maternal antibodies and immune cells)100 and in the maternal influence on the child’s microbiota. In the second trimester of pregnancy, human foetuses are exposed in utero to live bacteria, including staphylococci and lactobacilli, which can induce in vitro activation of memory T cells in lymph nodes91, suggesting that in utero microbial exposure contributes to acquisition of T cell immune memory and priming before birth. Supporting the hypothesis that infections in pregnancy can have subsequent effects on immunity and predisposition to inflammatory disorders in offspring, evidence from a murine model has shown that maternally restricted and transient infection with attenuated Yersinia pseudotuberculosis is associated with elevation of numbers of TH17 cells in the lamina propria of adult offspring, which have enhanced reactivity to microbiota and susceptibility to mucosal inflammation101. This tissue imprinting is dependent on IL-6, and in the presence of IL-6 it is independent of maternal microbiota.
The adaptive immune system of the fetus and the child gradually acquires the ability to respond to specific antigenic challenges. By nature, the naive immune system harbours tolerogenic cells, including naive dendritic cells and regulatory T (Treg) cells. Priming of the immune system reshapes the immune response and the predisposition to later inflammatory arthropathies. This influence starts in utero with exposure to maternal microbiota, and continues with breastfeeding and with environmental exposures. Altogether, these factors are responsible for large modifications of the microbiota during growth, especially in the gut, and for the subsequent activation of the immune system, potentially triggering the emergence of arthritis in children.
Breastfeeding affects a child’s microbiota102,103. Milk contains immunomodulatory components, such as secretory IgA, which shape the intestinal microbiota, favouring beneficial bacteria. Milk also contains cytokines, such as IL-6, IL-10, transforming growth factor β1 (TGFβ1) and TGFβ2, which affect the infant’s microbiota103. Another potential mechanism for specific maternal influence on the offspring’s risk of developing autoimmune responses involves maternal–fetal microchimerism, resulting from the bidirectional passage of fetal and maternal cells across the placenta. Studies of maternal microchimerism in relation to juvenile autoimmune diseases104,105,106 have not yet demonstrated any contribution to a loss of tolerance, although evidence specific to JIA or RA has not yet been published.
Regulation of autoimmune responses
Naive T cells from healthy babies retain a propensity to become Treg cells, while they develop the capacity to differentiate into TH17 cells, until the age of at least 12 months. In this way, as immunity against pathogens develops91, the immune system maintains a regulatory profile107. Moreover, besides naturally occurring thymus-derived Treg cells108, Treg cells can be induced in the periphery from naive T cells in the presence of TGFβ109.
Patients with JIA have T cell-compartment dysregulation. T cell diversity (especially in relation to Treg cells) in patients with refractory JIA seems to be restricted when compared with healthy individuals110, and conserved and pathogenic T cell clones can be found in both blood and synovial fluid47,110,111. Results from seminal studies that followed the reconstitution of the T cell compartment after a ‘hard reset’ of the immune system in patients severely affected by JIA and undergoing autologous stem cell transplantation suggested functional renewal of Treg cells and T cell receptor diversification as potential mechanisms underlying the beneficial effect of this treatment in a substantial number of the patients112,113. These observations suggest a role for a diverse (naturally occurring and induced) Treg cell repertoire (early in life) in prevention of the development of chronic autoimmune responses (Fig. 2).
Microbiota in health and disease
The gut is the main interface of the immune system with the environment, and the gut microbiota affect health and disease. Its composition is subject to important inter-individual and intra-individual variability114. Notably, in conditions of systemic immune dysregulation, gut, respiratory and oral microbiota are largely interconnected with the immune system115,116,117 (Fig. 2). From experimental models in chronic inflammatory bowel disease, it seems clear that the microbiota influence immune activation, but also that chronic inflammation in turn shapes the gut microbiota and contributes to dysbiosis118. In RA, evidence suggests that the transcriptome of cell infiltrate from highly inflamed synovial tissue reflects synovial macrophage activation by microbial (bacterial and fungal) sources, potentially originating from the gut119. Other results from studies in humans have emphasized the pronounced effect of age on microbiota, and the existence of a threshold between child and adult microbiota in the first years of life120,121,122,123. Such modifications may account for differences in the behaviour of the immune system in immune arthritis associated with ageing.
(Epi)genetic–environmental interactions
As discussed in relation to the ‘critical window in (epi)genetics’ hypothesis, human immune arthritis has a significant (epi)genetic component, which is suspected to be differentially modulated in adults and in children, but this component is not sufficient to explain all major age-related differences. In addition to genetic and epigenetic factors, multiple parameters may contribute to the differences between adults and children, such as (for example) parental circumstances, social class, lifestyle, nutrition and diet, development stage, pharmacology and cultural behaviours. These interactions are multidirectional and complex, and the molecular and clinical integration of these factors is not yet fully understood. However, age-related differences in disease expression suggest that the gene–environment interaction is unlikely to drive arthritis equally or similarly across the lifespan of an individual. Conceptually, priming of the immune system early in life must be one of the most important factors that affect autoimmune responses in immune-mediated arthritis.
Burden-accumulation hypothesis
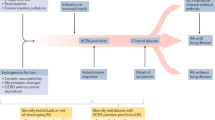
This hypothesis centralizes the accumulated burden of both environmental and host-related factors in ageing and their consequences for shaping and refining immune responses, potentially resulting in chronic immune-mediated arthritis at different ages. Growing and then ageing are also associated with progressive joint ‘priming’, in which the experience of articular challenges (re)models the local tissue and potentially the articular immune response. This phenomenon results from mechanical stress or damage, microbial challenges or any other extra-articular influence, and it suggests a heterogeneity in the development of arthritis that depends on the baseline status of the joint (‘naive’ versus ‘primed’) (Fig. 3).
Proposed (age-dependent) factors determining arthritis ‘heterogeneity’, depending on the baseline status of the joint (‘naive’ versus ‘primed’). In naive and in primed joints, some factors are presumed to have a dampening influence on joint inflammation, and similarly, in naive and in primed joints, other factors are thought to have a perpetuating influence on inflammation. T(RM) cells, tissue-resident memory T cells.
Oestrogens
Host-related factors participate in the generation of an inflammatory burden in IMIDs. Women usually have stronger adaptive immune responses than men and greater susceptibility to autoimmunity, as observed after puberty in relation to RA and SLE124. This sex bias is not observed in enthesitis-related arthritis (ERA)-JIA or its adult counterpart spondyloarthritis. Underlying mechanisms for the sex bias involve the sex steroid hormone 17β-oestradiol (E2), acting through oestrogen receptor-α (ERα) in plasmacytoid dendritic cells to enhance the type I interferon response to activation of TLR7 and TLR9, so that this response is stronger in women than in men125. Oestrogens also enhance the CD4+ effector T cell response126 and increase B cell activation and autoantibody production127. Notably, the TLR7 gene is located on the X chromosome. In women, one of the two X chromosomes in each cell is physiologically inactivated. However, as recently demonstrated, TLR7 can escape from X-inactivation in B cells and myeloid cells in women and individuals with Klinefelter syndrome (47, XXY)128. This biallelic TLR7 expression is functionally responsible for enhancement of TLR7-dependent B cell proliferation and immunoglobulin class-switching. These observations might explain female susceptibility to SLE and potentially to other IMIDs.
Obesity and autoimmune inflammation
Obesity is a relevant comorbidity with the potential to contribute to chronic inflammation, as a result of the accumulation in adipose tissue of pro-inflammatory immune cells such as M1 macrophages, mast cells, neutrophils and CD4+ and CD8+ T cells129. The involvement of some of these cell types in IMIDs raises the question of whether common or intersecting pathways are associated with obesity and IMIDs.
The association of psoriasis and PsA with obesity suggests the existence of a causal relationship. Notably, a genetic risk score incorporating 97 SNPs can account for ~2.7% of variation in adult BMI130. The relationship between BMI and psoriasis was analysed in 753,421 patients from the UK Biobank and the Nord-Trøndelag Health Study131, using a Mendelian randomization approach based on the 97-SNP genetic risk score as an optimized surrogate for BMI. This analysis showed that a 1 kg/m² increase in BMI is associated with a 9% increase in the odds of psoriasis. This causality link was unidirectional, with no strong evidence of an effect of psoriasis genetic risk on BMI. However, a limitation of this approach was that BMI SNPs are a stronger instrument for adult BMI than for child BMI132. Genetic background and biological pathways involved in adiposity and obesity only partly overlap between adults and children. Thus, direct transposition of adult analysis tools to childhood is still not straightforward.
The differential effects of smoking
The relationship between smoking and HLA-DRB1 SE alleles is a well-described gene–environment interaction with regard to the development of ACPA-positive RA133,134. The current dogma is that individuals carrying HLA-DRB1 SE alleles are dose-dependently predisposed to developing a rheumatoid self-reactivity to citrullinated peptides12, which are more prominent in lungs of smokers than in those of non-smokers. Another major genetic risk factor that is associated with ACPA-positive RA is the PTPN22 polymorphism encoding the R620W substitution. Data from the EIRA, North American RA Consortium and Leiden Early Arthritis Clinic studies have highlighted complex gene–gene (HLA-DRB1 SE–PTPN22) and gene–environment (HLA-DRB1 SE–smoking and PTPN22–smoking) interactions. Notably, these interactions are associated with seropositive, but not seronegative, RA135.
An illustration of how environmental factors can act upstream of gene regulation and disease phenotype is epigenetic modulation induced by smoking. Results of a meta-analysis of genome-wide DNA methylation highlight the association between smoking and DNA methylation136. Smoking can either increase or reduce CpG methylation. CpGs in DNA derived from whole blood, CD4+ T cells or monocytes are differentially methylated in current versus never smokers. This pattern of alteration persists in former smokers (defined as having stopped ≥12 months prior to the blood test) within 5 years of stopping smoking for most CpGs. A minority of altered CpGs persist 30 years after smoking cessation, and some of these CpGs are associated with genes that are potentially involved in RA pathogeny (such as AHRR)137,138,139. Interestingly, enrichment in the smoking methylation phenotype is also observed in genome-wide association studies in RA, osteoporosis and inflammatory bowel disease140. These findings suggest a functional relevance of smoking-related CpG methylation patterns in arthritis, especially in RA.
Microbiota
Microbiota affect many aspects of human physiology, and their composition varies throughout the lifespan of an individual. The first 3 years of life are critical for mature microbiota constitution, and might represent a window of opportunity for microbial modulation120,121,122. In adulthood and old age, diet, lifestyle and medication affect the microbiota123.
Microbiota are heritable from the mother, and this vertical transmission is influenced by the birth mode141. Other factors that affect microbiota transmission include maternal health status and lifestyle during pregnancy, antibiotic use around birth, geographical location, family environment, type of feeding, duration of lactation and use of antibiotics in infancy142. Microbiota composition varies according to infant body sites and age143. Importantly, inheritance of particular species can affect later pathological outcomes such as the development of obesity (with specific changes in Christensenella minuta, Akkermansia muciniphila, Methanobrevibacter smithii and Blautia spp. operational taxonomic units)144. Disturbances in the microbiota early in life might be a risk factor for the development of autoimmune responses and disease later on. Results from two large case–control studies (in the UK and Finland) have identified increased risk of the development of JIA in children with cow’s milk allergies145 and in children with antibiotic use early in life146,147. The association with antibiotic use was dose-dependent, had the strongest association with antibiotic use in the first 12 months of life and did not substantially change when adjusted for the number and type of infections. In a Dutch–Italian inception-cohort study of patients with new-onset JIA, signs of dysbiosis were found in the gut microbiota of patients with JIA relative to healthy individuals (siblings or age-matched, sex-matched schoolmates)148.
The human virome (as part of the microbiome) clearly represents key modulators of human health149,150, especially for the emergence of autoimmunity, as exemplified by the association between Epstein–Barr virus (EBV) and the onset of multiple sclerosis151. The proposed mechanisms for EBV-driven development of multiple sclerosis are molecular mimicry, B cell transformation and induction of B cell trafficking to the central nervous system152. The association with EBV might also be applicable to autoimmune arthritis, such as RA. The antibody response to EBV (IgG against the viral early antigen) is specifically elevated in the serum of patients with preclinical RA, and its increase correlates with rheumatoid factor seroconversion153, suggesting an EBV reactivation in preclinical RA and a role for RA development.
From development to senescence
Modifications in the behaviour of the immune system are likely to account, at least in part, for differences between adult and childhood IMIDs. In the first year of life, naive T cells will encounter a large variety of non-self antigens as they are exposed to an increasing variety of both food antigens and pathogens (as they go through their first infectious episodes). The developing immune system in healthy individuals has developed mechanisms to avoid autoimmune responses and aberrant response to non-pathogenic non-self antigens.
Immunosenescence
During ageing, the immune system undergoes profound changes in a process called immunosenescence, which is responsible for susceptibility to infection, impairment of antivaccine responses, a weakened defence against malignancies and impaired wound repair154. At the cellular level, immunosenescence is associated with reduced maintenance of immune and haematopoietic cells by haematopoietic stem cells, the output of which is reduced in diversity and functionally biased155. In a longitudinal study of blood samples from 135 healthy adults over a period of 9 years by mass cytometry, dynamic changes in circulating immune-cell subpopulations were assessed, enabling identification of a gene set, the expression of which correlated with scores for the trajectory of immune ageing (IMM-AGE)156. The IMM-AGE score, which was a summary of the rates of change of numbers of immune cells in an individual, correlated functionally with all-cause mortality, suggesting that it is a better measure than chronological age for assessment of immune ageing.
RA is associated with several extra-articular features that shape (or reshape) the immune response. These features include premature immunosenescence, which might result from genomic instability linked to DNA damage, telomere shortening, impaired autophagy and protein homeostasis, mitochondrial dysfunction (with mitochondrial DNA mutations and production of reactive oxygen species) and/or stem cell exhaustion157. In the context of intense rheumatoid expansion of the immune cells (also termed advanced replicative stress), all these mechanisms merge to lead to a premature immunosenescence with altered function of leukocytes (especially T cells)158. Interestingly, in cohorts with suspected early RA, individuals with younger and older age of onset differ in terms of genetic background, clinical presentation and prognosis. Older age at RA onset is usually associated with lower rates of positivity for rheumatoid factor and ACPA, lower rate of remission, higher radiographic progression and lower functional score during follow-up159,160. These differences may be partly explained by immunosenescence.
Viral stimuli have long been suspected to trigger arthritis, especially RA. In a gerontological context, human cytomegalovirus (HCMV) infection accelerates some features of immunosenescence, notably by promoting the expansion of senescent T cells (CD28−)161,162. Interestingly, in addition to immunosenescence, RA is associated with age-related diseases such as cardiovascular disease. HCMV and HCMV-specific T cells are also involved in cardiovascular disease, a typical age-related condition163,164. More studies are necessary to understand the role of chronic viral infection during clinical progression in RA165,166,167. Seroprevalence for HCMV infection ranges from 40% to almost 100%, with higher prevalence associated with female gender, low socioeconomic status and specific geographic locations (such as South America, Africa, Asia and the Middle East)168,169,170. Moreover, HCMV seroprevalence increases with age, with estimated positivity of 21.5% in children at ages 1–2 years and 32.0% at ages 14–17 years171. Given its ability to establish a lifelong latent infection, and to reshape the immune response for immune escape, a reasonable hypothesis is that HCMV represents one of the triggering factors for chronic inflammatory arthritis. Differential effects on T cell effector subsets are observed in patients with JIA and in healthy children following HCMV infection172.
Inflammageing
Ageing immune cells contribute to age-related pathological conditions such as osteoarthritis, atherosclerosis and neurodegenerative diseases. In addition, the pro-inflammatory burden, carried by ageing cells and tissues, and resulting in age-related chronic comorbidities (also termed inflammageing), occurs earlier in IMIDs such as RA173,174. This phenomenon, extensively reviewed previously175, is associated with high levels of pro-inflammatory cytokines, especially IL-6, and might contribute to the differentiation between adult disease and the onset of disease in children, in whom the accumulated inflammatory burden is likely to be a lot less.
Conclusions
Improved understanding of age-related mechanisms shaping the immune system could help to bring together adult and paediatric perspectives on the development of immune-mediated arthritis. In this Review, we have discussed several age-dependent physiological mechanisms that modulate disease presentation, by presenting three (not mutually exclusive) hypotheses. These mechanisms are potentially synergistic and are likely to explain some of the differences and commonalities between age groups. By defining these hypotheses, we aim to encourage a more unified view of the diagnosis and treatment of both childhood and adult arthritis. Notably, other Reviews have discussed the genetic classification of primary arthritis9,26, supporting the premise that distinct biological signatures will enable the partitioning of patients into groups that are amenable to mechanism-based intervention15. New classification systems for juvenile and adult inflammatory arthritis can help to unite clinical perspectives. However, classifications do not (and are not meant to) explain what lies beneath clinical phenotypes. As discussed here, knowledge of the underlying genetic influences certainly helps to clarify important risk factors in IMIDs, but it does not tell the full story. Further understanding is necessary of how, against a specific genetic background, the immune system interacts with the environment, leading to epigenetic changes and the ensuing autoimmune response. In this paradigm, age is an important, but mostly overlooked, factor. When and how an environmental trigger activates the developing immune system determines the ultimate immune response. The first step in unravelling this complex, multidimensional process would be to build a comprehensive framework to describe the development of adaptive and innate immunity over time, as was done long ago for normal growth and development176. Although such an endeavour would be complex and challenging, the rewards would be considerable, in terms of better understanding of the emergence of IMIDs, and possibly also the identification of the potential for truly innovative interventions. Following on from studies of genetics, epigenetics and environmental interactions, inflammageing could be the next frontier in inflammatory arthritis. Whatever else, the hope is that the molecular medicine revolution can provide effective solutions for the treatment of IMIDs in young and old alike.
References
Gotzinger, F. et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc. Health 4, 653–661 (2020).
Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 27, 28–33 (2021). Comprehensive review of immune determinants of COVID-19 disease.
Sancho-Shimizu, V. et al. SARS-CoV-2-related MIS-C: a key to the viral and genetic causes of Kawasaki disease? J. Exp. Med. 218, e20210446 (2021).
Hysa, E. et al. Immune system activation in polymyalgia rheumatica: which balance between autoinflammation and autoimmunity? A systematic review. Autoimmun. Rev. 21, 102995 (2022).
Samson, M. et al. Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis Rheum. 64, 3788–3798 (2012).
Dejaco, C. et al. NKG2D stimulated T-cell autoreactivity in giant cell arteritis and polymyalgia rheumatica. Ann. Rheum. Dis. 72, 1852–1859 (2013).
Kuan, V. et al. A chronological map of 308 physical and mental health conditions from 4 million individuals in the English National Health Service. Lancet Digital Health 1, e63–e77 (2019). Comprehensive analysis of disease occurrence across ages.
No authors listed. Criteria for the classification of juvenile rheumatoid arthritis. Bull. Rheum. Dis. 23, 712–719 (1972).
Nigrovic, P. A., Martinez-Bonet, M. & Thompson, S. D. Implications of juvenile idiopathic arthritis genetic risk variants for disease pathogenesis and classification. Curr. Opin. Rheumatol. 31, 401–410 (2019).
Consolaro, A. et al. Phenotypic variability and disparities in treatment and outcomes of childhood arthritis throughout the world: an observational cohort study. Lancet Child Adolesc. Health 3, 255–263 (2019).
Petty, R. E. et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol. 31, 390–392 (2004).
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 4, 18001 (2018).
Stoll, M. L. & Nigrovic, P. A. Subpopulations within juvenile psoriatic arthritis: a review of the literature. Clin. Dev. Immunol. 13, 377–380 (2006).
Debrach, A. C. et al. Comparison of paediatric and adult classification criteria in juvenile idiopathic arthritis during the transition from paediatric to adult care. Joint Bone Spine 88, 105047 (2021).
Nigrovic, P. A. et al. Biological classification of childhood arthritis: roadmap to a molecular nomenclature. Nat. Rev. Rheumatol. 17, 257–269 (2021). Proposes a roadmap for molecular classification of childhood arthritis.
Eyre, S., Orozco, G. & Worthington, J. The genetics revolution in rheumatology: large scale genomic arrays and genetic mapping. Nat. Rev. Rheumatol. 13, 421–432 (2017).
Stastny, P. Mixed lymphocyte cultures in rheumatoid arthritis. J. Clin. Invest. 57, 1148–1157 (1976).
Gregersen, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis. an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30, 1205–1213 (1987).
Begovich, A. B. et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 75, 330–337 (2004).
Hinks, A. et al. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat. Genet. 45, 664–669 (2013).
Forre, O., Dobloug, J. H., Hoyeraal, H. M. & Thorsby, E. HLA antigens in juvenile arthritis. Genetic basis for the different subtypes. Arthritis Rheum. 26, 35–38 (1983).
Hinks, A. et al. Fine-mapping the MHC locus in juvenile idiopathic arthritis (JIA) reveals genetic heterogeneity corresponding to distinct adult inflammatory arthritic diseases. Ann. Rheum. Dis. 76, 765–772 (2017).
Hollenbach, J. A. et al. Juvenile idiopathic arthritis and HLA Class I and Class II interactions and age-at-onset effects. Arthritis Rheum. 62, 1781–1791 (2010).
Hinks, A. et al. Association between the PTPN22 gene and rheumatoid arthritis and juvenile idiopathic arthritis in a UK population: further support that PTPN22 is an autoimmunity gene. Arthritis Rheum. 52, 1694–1699 (2005).
Lins, T. C., Vieira, R. G., Grattapaglia, D. & Pereira, R. W. Allele and haplotype frequency distribution in PTPN22 gene across variable ethnic groups: implications for genetic association studies for autoimmune diseases. Autoimmunity 43, 308–316 (2010).
Nigrovic, P. A., Raychaudhuri, S. & Thompson, S. D. Review: genetics and the classification of arthritis in adults and children. Arthritis Rheumatol. 70, 7–17 (2018). Proposes a new paradigm for classification of arthritides.
Stahl, E. A. et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Genet. 42, 508–514 (2010).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 447, 661–678 (2007).
Macgregor, A. J. et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 43, 30–37 (2000).
Plenge, R. M. et al. TRAF1–C5 as a risk locus for rheumatoid arthritis — a genomewide study. N. Engl. J. Med. 357, 1199–1209 (2007).
Okada, Y. et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506, 376–381 (2014).
Okada, Y. et al. Risk for ACPA-positive rheumatoid arthritis is driven by shared HLA amino acid polymorphisms in Asian and European populations. Hum. Mol. Genet. 23, 6916–6926 (2014).
Viatte, S., Plant, D. & Raychaudhuri, S. Genetics and epigenetics of rheumatoid arthritis. Nat. Rev. Rheumatol. 9, 141–153 (2013).
Angiolilli, C. et al. New insights into the genetics and epigenetics of systemic sclerosis. Nat. Rev. Rheumatol. 14, 657–673 (2018).
Holliday, R. & Pugh, J. E. DNA modification mechanisms and gene activity during development. Science 187, 226–232 (1975).
Morgan, H. D., Sutherland, H. G. E., Martin, D. I. K. & Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 23, 314–318 (1999).
Waterland, R. A. & Jirtle, R. L. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 23, 5293–5300 (2003).
Willard, H., Brown, C., Carrel, L., Hendrich, B. & Miller, A. Epigenetic and chromosomal control of gene expression: molecular and genetic analysis of X chromosome inactivation. Cold Spring Harb. Symp. Quant. Biol. 58, 315–322 (1995).
Strickland, F. M. et al. Environmental exposure, estrogen and two X chromosomes are required for disease development in an epigenetic model of lupus. J. Autoimmun. 38, J135–143 (2012).
Laird, P. W. Principles and challenges of genome-wide DNA methylation analysis. Nat. Rev. Genet. 11, 191–203 (2010).
Cedar, H. & Bergman, Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat. Rev. Genet. 10, 295–304 (2009).
Karouzakis, E., Gay, R. E., Michel, B. A., Gay, S. & Neidhart, M. DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 60, 3613–3622 (2009).
Nakano, K., Whitaker, J. W., Boyle, D. L., Wang, W. & Firestein, G. S. DNA methylome signature in rheumatoid arthritis. Ann. Rheum. Dis. 72, 110–117 (2013).
Friscic, J. et al. The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity 54, 1002–1021 e1010 (2021).
Liu, Y. et al. Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nat. Biotechnol. 31, 142–147 (2013).
Webster, A. P. et al. Increased DNA methylation variability in rheumatoid arthritis-discordant monozygotic twins. Genome Med. 10, 64 (2018).
Mijnheer, G. et al. Conserved human effector Treg cell transcriptomic and epigenetic signature in arthritic joint inflammation. Nat. Commun. 12, 2710 (2021). Characterizes tissue (synovial fluid) specific effector Treg signatures in arthritic joint inflammation.
Peeters, J. G. et al. Inhibition of super-enhancer activity in autoinflammatory site-derived T cells reduces disease-associated gene expression. Cell Rep. 12, 1986–1996 (2015).
Peeters, J. G. C., Vastert, S. J., van Wijk, F. & van Loosdregt, J. Review: enhancers in autoimmune arthritis: implications and therapeutic potential. Arthritis Rheumatol. 69, 1925–1936 (2017).
Zhao, M. et al. Epigenetics and SLE: RFX1 downregulation causes CD11a and CD70 overexpression by altering epigenetic modifications in lupus CD4+ T cells. J. Autoimmun. 35, 58–69 (2010).
Sava, G. P., Fan, H., Coombes, R. C., Buluwela, L. & Ali, S. CDK7 inhibitors as anticancer drugs. Cancer Metastasis Rev. 39, 805–823 (2020).
Bandukwala, H. S. et al. Selective inhibition of CD4+ T-cell cytokine production and autoimmunity by BET protein and c-Myc inhibitors. Proc. Natl Acad. Sci. USA 109, 14532–14537 (2012).
Mele, D. A. et al. BET bromodomain inhibition suppresses TH17-mediated pathology. J. Exp. Med. 210, 2181–2190 (2013).
Richardson, B., Kahn, L., Lovett, E. J. & Hudson, J. Effect of an inhibitor of DNA methylation on T cells. I. 5-Azacytidine induces T4 expression on T8+T cells. J. Immunol. 137, 35–39 (1986).
Födinger, M., Hörl, W. H. & Sunder-Plassmann, G. Molecular biology of 5,10-methylenetetrahydrofolate reductase. J. Nephrol. 13, 20–33 (2000).
Ellis, J. A. et al. Genome-scale case-control analysis of CD4+ T-cell DNA methylation in juvenile idiopathic arthritis reveals potential targets involved in disease. Clin. Epigenetics 4, 20 (2012).
Kim, Y.-I., Logan, J. W., Mason, J. B. & Roubenoff, R. DNA hypomethylation in inflammatory arthritis: reversal with methotrexate. J. Lab Clin. Med. 128, 165–172 (1996).
Carini, C. et al. Chromosome conformation signatures define predictive markers of inadequate response to methotrexate in early rheumatoid arthritis. J. Transl. Med. 16, 18 (2018).
Dolinoy, D. C., Das, R., Weidman, J. R. & Jirtle, R. L. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediatr. Res. 61, 30R–37R (2007).
Waseem Bihaqi, S., Schumacher, A., Maloney, B., Lahiri, D. K. & Zawia, N. Do epigenetic pathways initiate late onset Alzheimer disease (LOAD): towards a new paradigm. Curr. Alzheimer Res. 9, 574–588 (2012).
Wang, S.-C., Oelze, B. & Schumacher, A. Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS One 3, e2698 (2008).
Bjornsson, H. T. Intra-individual change over time in DNA methylation with familial clustering. JAMA 299, 2877 (2008).
Sen, E. S. & Ramanan, A. V. Juvenile idiopathic arthritis-associated uveitis. Clin. Immunol. 211, 108322 (2020).
Yokota, S. et al. Longterm safety and effectiveness of the anti-interleukin 6 receptor monoclonal antibody tocilizumab in patients with systemic juvenile idiopathic arthritis in Japan. J. Rheumatol. 41, 759–767 (2014).
Giannini, E. H. et al. Longitudinal analysis of HLA associated risks for iridocyclitis in juvenile rheumatoid arthritis. J. Rheumatol. 18, 1394–1397 (1991).
Melin-Aldana, H. et al. Human leukocyte antigen-DRB1*1104 in the chronic iridocyclitis of pauciarticular juvenile rheumatoid arthritis. J. Pediatr. 121, 56–60 (1992).
Wildschutz, L. et al. Transcriptomic and proteomic analysis of iris tissue and aqueous humor in juvenile idiopathic arthritis-associated uveitis. J. Autoimmun. 100, 75–83 (2019).
Ramanan, A. V. et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N. Engl. J. Med. 376, 1637–1646 (2017).
Roche, D., Badard, M., Boyer, L., Lafforgue, P. & Pham, T. Incidence of anterior uveitis in patients with axial spondyloarthritis treated with anti-TNF or anti-IL17A: a systematic review, a pairwise and network meta-analysis of randomized controlled trials. Arthritis Res. Ther. 23, 192 (2021).
Eder, L. et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 68, 915–923 (2016).
Belasco, J. et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol. 67, 934–944 (2015).
Veale, D. J. & Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 391, 2273–2284 (2018).
Chen, L. et al. Skin expression of IL-23 drives the development of psoriasis and psoriatic arthritis in mice. Sci. Rep. 10, 8259 (2020).
Diani, M. et al. Increased frequency of activated CD8+ T cell effectors in patients with psoriatic arthritis. Sci. Rep. 9, 10870 (2019).
Leijten, E. F. et al. Tissue-resident memory CD8+ T cells from skin differentiate psoriatic arthritis from psoriasis. Arthritis Rheumatol. 73, 1220–1232 (2021).
Charras, A. et al. DNA methylation patterns in CD8+ T cells discern psoriasis from psoriatic arthritis and correlate with cutaneous disease activity. Front. Cell Dev. Biol. 9, 746145 (2021).
Gelfand, J. M. et al. The risk of mortality in patients with psoriasis. Arch. Dermatol. 143, 7 (2007).
Prodanovich, S. et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch. Dermatol. 145, 4 (2009).
Boehncke, W.-H. & Schön, M. P. Psoriasis. Lancet 386, 983–994 (2015).
Devrimci-Ozguven, H., Kundakci, T. N., Kumbasar, H. & Boyvat, A. The depression, anxiety, life satisfaction and affective expression levels in psoriasis patients. J. Eur. Acad. Dermatol. Venereol. 14, 267–271 (2000).
Dougados, M. et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann. Rheum. Dis. 73, 62–68 (2014).
Fakra, E. & Marotte, H. Rheumatoid arthritis and depression. Jt. Bone Spine 88, 105200 (2021).
Sparks, J. A. et al. Depression and subsequent risk for incident rheumatoid arthritis among women. Arthritis Care Res. 73, 78–89 (2021).
Vallerand, I. A. et al. Depression as a risk factor for the development of rheumatoid arthritis: a population-based cohort study. RMD Open 4, e000670 (2018).
Carpenter, L. L. et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 35, 2617–2623 (2010).
Neufeld, K. M., Karunanayake, C. P., Maenz, L. Y. & Rosenberg, A. M. Stressful life events antedating chronic childhood arthritis. J. Rheumatol. 40, 1756–1765 (2013).
Rubinstein, T. B. et al. Adverse childhood experiences are associated with childhood-onset arthritis in a national sample of US youth: an analysis of the 2016 National Survey of Children’s Health. J. Pediatr. 226, 243–250.e2 (2020).
Bierhaus, A. et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl Acad. Sci. USA 100, 1920–1925 (2003).
Glaser, R. & Kiecolt-Glaser, J. K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 5, 243–251 (2005).
Henneke, P., Kierdorf, K., Hall, L. J., Sperandio, M. & Hornef, M. Perinatal development of innate immune topology. Elife 10, e67793 (2021).
Mishra, A. et al. Microbial exposure during early human development primes fetal immune cells. Cell 184, 3394–3409 e3320 (2021). Identified microbial influence during fetal life, priming the immune system.
Rechavi, E. et al. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci. Transl. Med. 7, 276ra225 (2015).
Krey, P. R., Cohen, A. S., Smith, C. B. & Finland, M. The human fetal synovium. Histology, fine structure and changes in organ culture. Arthritis Rheum. 14, 319–341 (1971).
Wynne-Roberts, C. R., Anderson, C. H., Turano, A. M. & Baron, M. Light- and electron-microscopic findings of juvenile rheumatoid arthritis synovium: comparison with normal juvenile synovium. Semin. Arthritis Rheum. 7, 287–302 (1978).
Pasquali-Ronchetti, I. et al. Aging of the human synovium: an in vivo and ex vivo morphological study. Semin. Arthritis Rheum. 21, 400–414 (1992).
Chang, M. H. & Nigrovic, P. A. Antibody-dependent and -independent mechanisms of inflammatory arthritis. JCI Insight 4, e125278 (2019).
Laver-Rudich, Z. & Silbermann, M. Cartilage surface charge. A possible determinant in aging and osteoarthritic processes. Arthritis Rheum. 28, 660–670 (1985).
Fearon, D. T. Regulation by membrane sialic acid of β1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc. Natl Acad. Sci. USA 75, 1971–1975 (1978).
Hiemstra, P. S. et al. Activation of complement by human serum IgA, secretory IgA and IgA1 fragments. Mol. Immunol. 25, 527–533 (1988).
Albrecht, M. & Arck, P. C. Vertically transferred immunity in neonates: mothers, mechanisms and mediators. Front. Immunol. 11, 555 (2020).
Lim, A. I. et al. Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science 373, eabf3002 (2021).
Westrom, B., Arevalo Sureda, E., Pierzynowska, K., Pierzynowski, S. G. & Perez-Cano, F. J. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol. 11, 1153 (2020).
Kalbermatter, C., Fernandez Trigo, N., Christensen, S. & Ganal-Vonarburg, S. C. Maternal microbiota, early life colonization and breast milk drive immune development in the newborn. Front. Immunol. 12, 683022 (2021).
Stevens, A. M. Do maternal cells trigger or perpetuate autoimmune diseases in children? Pediatr. Rheumatol. Online J. 5, 9 (2007).
Ye, Y. et al. Maternal microchimerism in muscle biopsies from children with juvenile dermatomyositis. Rheumatology 51, 987–991 (2012).
Artlett, C. M., Sassi-Gaha, S., Ramos, R. C., Miller, F. W. & Rider, L. G. Chimeric cells of maternal origin do not appear to be pathogenic in the juvenile idiopathic inflammatory myopathies or muscular dystrophy. Arthritis Res. Ther. 17, 238 (2015).
Dijkstra, K. K., Hoeks, S. B., Prakken, B. J. & de Roock, S. TH17 differentiation capacity develops within the first 3 months of life. J. Allergy Clin. Immunol. 133, 891–894.e5 (2014).
Sakaguchi, S. et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27 (2006).
Apostolou, I. et al. Peripherally induced Treg: mode, stability, and role in specific tolerance. J. Clin. Immunol. 28, 619–624 (2008).
Henderson, L. A. et al. Next-generation sequencing reveals restriction and clonotypic expansion of Treg cells in juvenile idiopathic arthritis. Arthritis Rheumatol. 68, 1758–1768 (2016). Identified restricted and clonotypic expansion of Treg cells in JIA.
Wehrens, E. J., Prakken, B. J. & van Wijk, F. T cells out of control — impaired immune regulation in the inflamed joint. Nat. Rev. Rheumatol. 9, 34–42 (2013).
de Kleer, I. et al. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood 107, 1696–1702 (2006).
Delemarre, E. M. et al. Autologous stem cell transplantation aids autoimmune patients by functional renewal and TCR diversification of regulatory T cells. Blood 127, 91–101 (2016).
Hall, A. B., Tolonen, A. C. & Xavier, R. J. Human genetic variation and the gut microbiome in disease. Nat. Rev. Genet. 18, 690–699 (2017).
Zhang, X. et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21, 895–905 (2015).
Vujkovic-Cvijin, I. et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 5, 193ra191 (2013).
Gracey, E. et al. Revisiting the gut-joint axis: links between gut inflammation and spondyloarthritis. Nat. Rev. Rheumatol. 16, 415–433 (2020).
Ni, J., Wu, G. D., Albenberg, L. & Tomov, V. T. Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584 (2017).
Smiljanovic, B. et al. Synovial tissue transcriptomes of long-standing rheumatoid arthritis are dominated by activated macrophages that reflect microbial stimulation. Sci. Rep. 10, 7907 (2020).
Rodríguez, J. M. et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26, 26050 (2015).
Saraswati, S. & Sitaraman, R. Aging and the human gut microbiota from correlation to causality. Front. Microbiol. 5, 764 (2015).
Yassour, M. et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 8, 343ra381 (2016). Highlights the critical role of early life in microbiota constitution.
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Desai, M. K. & Brinton, R. D. Autoimmune disease in women: endocrine transition and risk across the lifespan. Front. Endocrinol. 10, 265 (2019).
Seillet, C. et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 119, 454–464 (2012).
Maret, A. et al. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor α expression in hematopoietic cells. Eur. J. Immunol. 33, 512–521 (2003).
Tabor, D. E. & Gould, K. A. Estrogen receptor alpha promotes lupus in (NZBxNZW)F1 mice in a B cell intrinsic manner. Clin. Immunol. 174, 41–52 (2017).
Souyris, M. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 3, eaap8855 (2018).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015).
Budu-Aggrey, A. et al. Evidence of a causal relationship between body mass index and psoriasis: a Mendelian randomization study. PLoS Med. 16, e1002739 (2019).
Monnereau, C., Vogelezang, S., Kruithof, C. J., Jaddoe, V. W. V. & Felix, J. F. Associations of genetic risk scores based on adult adiposity pathways with childhood growth and adiposity measures. BMC Genet. 17, 120 (2016).
Klareskog, L. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA–DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 54, 38–46 (2006).
Padyukov, L. et al. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 50, 3085–3092 (2004).
Källberg, H. et al. Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am. J. Hum. Genet. 80, 867–875 (2007).
Joehanes, R. et al. Epigenetic signatures of cigarette smoking. Circ. Cardiovasc. Genet. 9, 436–447 (2016).
Cheng, L. & Qian, L. Aromatic hydrocarbon receptor provides a link between smoking and rheumatoid arthritis in peripheral blood mononuclear cells. Clin. Exp. Rheumatol. 37, 445–449 (2019).
Cheng, L., Qian, L., Wang, G.-S., Li, X.-M. & Li, X.-P. Genetic association of aromatic hydrocarbon receptor and its repressor gene polymorphisms with risk of rheumatoid arthritis in Han Chinese populations. Medicine 96, e6392 (2017).
Kazantseva, M. G., Highton, J., Stamp, L. K. & Hessian, P. A. Dendritic cells provide a potential link between smoking and inflammation in rheumatoid arthritis. Arthritis Res. Ther. 14, R208 (2012).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking — 50 years of progress: a report of the Surgeon General. (Centers for Disease Control and Prevention, 2014).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Reyman, M. et al. Effects of early-life antibiotics on the developing infant gut microbiome and resistome: a randomized trial. Nat. Commun. 13, 893 (2022).
Milani, C. et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81, e00036–17 (2017).
Beaumont, M. et al. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol. 17, 189 (2016).
Arvonen, M., Virta, L. J., Pokka, T., Kroger, L. & Vahasalo, P. Cow’s milk allergy in infancy and later development of juvenile idiopathic arthritis: a register-based case-control study. Am. J. Epidemiol. 186, 237–244 (2017).
Arvonen, M., Virta, L. J., Pokka, T., Kroger, L. & Vahasalo, P. Repeated exposure to antibiotics in infancy: a predisposing factor for juvenile idiopathic arthritis or a sign of this group’s greater susceptibility to infections? J. Rheumatol. 42, 521–526 (2015).
Horton, D. B. et al. Antibiotic exposure and juvenile idiopathic arthritis: a case-control study. Pediatrics 136, e333–343 (2015).
van Dijkhuizen, E. H. P. et al. Microbiome analytics of the gut microbiota in patients with juvenile idiopathic arthritis: a longitudinal observational cohort study. Arthritis Rheumatol. 71, 1000–1010 (2019).
Turnbaugh, P. J. et al. The human microbiome project. Nature 449, 804–810 (2007).
Wylie, K. M. et al. Metagenomic analysis of double-stranded DNA viruses in healthy adults. BMC Biol. 12, 71 (2014).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022).
Robinson, W. H. & Steinman, L. Epstein-Barr virus and multiple sclerosis. Science 375, 264–265 (2022).
Fechtner, S. et al. Antibody responses to Epstein-Barr virus in the preclinical period of rheumatoid arthritis suggest the presence of increased viral reactivation cycles. Arthritis Rheumatol. 74, 597–603 (2022).
Weyand, C. M. & Goronzy, J. J. Aging of the immune system. mechanisms and therapeutic targets. Ann. Am. Thorac. Soc. 13, S422–S428 (2016).
Keenan, C. R. & Allan, R. S. Epigenomic drivers of immune dysfunction in aging. Aging Cell 18, e12878 (2019).
Alpert, A. et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 25, 487–495 (2019). Models the trajectory for immune aging.
Chalan, P., van den Berg, A., Kroesen, B. J., Brouwer, L. & Boots, A. Rheumatoid arthritis, immunosenescence and the hallmarks of aging. Curr. Aging Sci. 8, 131–146 (2015).
Bauer, M. E. Accelerated immunosenescence in rheumatoid arthritis: impact on clinical progression. Immun. Ageing 17, 6 (2020).
Arnold, M. B. et al. Are there differences between young- and older-onset early inflammatory arthritis and do these impact outcomes? An analysis from the CATCH cohort. Rheumatology 53, 1075–1086 (2014).
Krams, T. et al. Effect of age at rheumatoid arthritis onset on clinical, radiographic, and functional outcomes: The ESPOIR cohort. Jt. Bone Spine 83, 511–515 (2016).
Bano, A. et al. CD28null CD4 T-cell expansions in autoimmune disease suggest a link with cytomegalovirus infection. F1000Research 8, F1000 Faculty Rev-327 (2019).
Olsson, J. et al. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121, 187–201 (2000).
Pera, A. et al. CD28null pro-atherogenic CD4 T-cells explain the link between CMV infection and an increased risk of cardiovascular death. Theranostics 8, 4509–4519 (2018).
Yu, H. T. et al. Arterial stiffness is associated with cytomegalovirus-specific senescent CD8+ T cells. J. Am. Heart Assoc. 6, e006535 (2017).
Petersen, L. E. et al. Characterization of senescence biomarkers in rheumatoid arthritis: relevance to disease progression. Clin. Rheumatol. 38, 2909–2915 (2019).
Rauwel, B. et al. Inhibition of osteoclastogenesis by the RNA-binding protein QKI5: a novel approach to protect from bone resorption. J. Bone Miner. Res. 35, 753–765 (2020).
Rauwel, B. et al. Reduced progression of bone erosion in cytomegalovirus seropositive rheumatoid arthritis patients. Arthritis Res. Ther. 22, 13 (2020).
Bate, S. L., Dollard, S. C. & Cannon, M. J. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin. Infect. Dis. 50, 1439–1447 (2010).
Cannon, M. J., Schmid, D. S. & Hyde, T. B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 20, 202–213 (2010).
Zuhair, M. et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev. Med. Virol. 29, e2034 (2019).
Voigt, S., Schaffrath Rosario, A. & Mankertz, A. Cytomegalovirus seroprevalence among children and adolescents in Germany: data from the German Health Interview and Examination Survey for Children and Adolescents (KiGGS), 2003–2006. Open Forum Infect. Dis. 3, ofv193 (2016).
Prelog, M. et al. Indications for a disturbed peripheral T-cell homeostasis in juvenile idiopathic arthritis (JIA): absent expansion of CD28 T-cells and no decrease of naive T-cells in cytomegalovirus-positive patients with JIA. J. Rheumatol. 35, 520–527 (2008).
Li, Y., Goronzy, J. J. & Weyand, C. M. DNA damage, metabolism and aging in pro-inflammatory T cells. Exp. Gerontol. 105, 118–127 (2018).
Li, Y. et al. The DNA repair nuclease MRE11A functions as a mitochondrial protector and prevents T cell pyroptosis and tissue inflammation. Cell Metab. 30, 477–492.e6 (2019).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Yeo, J. G. et al. The extended polydimensional immunome characterization (EPIC) web-based reference and discovery tool for cytometry data. Nat. Biotechnol. 38, 679–684 (2020). Provides a comprehensive template for analysis of the immune system across ages.
Acknowledgements
Y.D. is affiliated to the Toulouse Institute for Infectious and Inflammatory Diseases (INFINITY), INSERM U1291, Toulouse, France. S.J.V. acknowledges grant support from the Dutch Arthritis Foundation (LLP10) to his department.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks M. Chang, P. Nigrovic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Degboe, Y., Vastert, S.J., Prakken, B.J. et al. How does age determine the development of human immune-mediated arthritis?. Nat Rev Rheumatol 18, 501–512 (2022). https://doi.org/10.1038/s41584-022-00814-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00814-3