Abstract
Background
The combination of polymyxins, meropenem, and sulbactam demonstrated efficacy against multi-drug-resistant bacillus Acinetobacter baumannii. These three antibiotics are commonly used against major blood, skin, lung, and heart muscle infections.
Objective
The objective of this study was to predict drug disposition and extrapolate the efficacy in these tissues using a physiologically based pharmacokinetic modeling approach that linked drug exposures to their target pharmacodynamic indices associated with antimicrobial activities against A. baumannii.
Methods
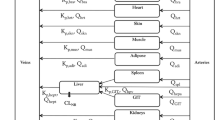
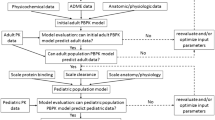
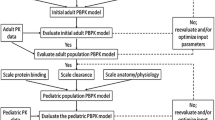
An adult physiologically based pharmacokinetic model was developed for meropenem, colistin, and sulbactam and scaled to pediatrics accounting for both renal and non-renal clearances. The model reliability was evaluated by comparing simulated plasma and tissue drug exposures to observed data. Target pharmacodynamic indices were used to evaluate whether pediatric and adult dosing regimens provided sufficient coverage.
Results
The modeled plasma drug exposures in adults and pediatric patients were consistent with reported literature data. The mean fold errors for meropenem, colistin, and sulbactam were in the range of 0.710–1.37, 0.981–1.47, and 0.647–1.39, respectively. Simulated exposures in the blood, skin, lung, and heart were consistent with reported penetration rates. In a virtual pediatric population aged from 2 to < 18 years, the interpretive breakpoints were achieved in 85–90% of subjects for their targeted pharmacodynamic indices after administration of pediatric dosing regimens consisting of 30 mg/kg of meropenem, and 40 mg/kg of sulbactam three times daily as a 3-h or continuous infusion and 5 mg/kg/day of colistin base activity.
Conclusions
The physiologically based pharmacokinetic modeling supports pediatric dosing regimens of meropenem/colistin/sulbactam in a co-administration setting against infections in the blood, lung, skin, and heart tissues due to A. baumannii.





Similar content being viewed by others
References
Oo C, Sy SKB. Learning and augmenting natural processes: potential means of combating antimicrobial resistance from a drug R&D perspective. Drug Discov Today. 2020;25(1):1–3.
Brown JM, Dorman DC, Roy LP. Acute renal failure due to overdosage of colistin. Med J Aust. 1970;2(20):923–4.
Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. Adverse effects of sodium colistimethate: manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med. 1970;72(6):857–68.
Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(6):1953–8.
Zhao M, Wu XJ, Fan YX, Zhang YY, Guo BN, Yu JC, et al. Pharmacokinetics of colistin methanesulfonate (CMS) in healthy Chinese subjects after single and multiple intravenous doses. Int J Antimicrob Agents. 2018;51(5):714–20.
Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol. 2014;5:643.
Qureshi ZA, Hittle LE, O’Hara JA, Rivera JI, Syed A, Shields RK, et al. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis. 2015;60(9):1295–303.
Sopirala MM, Pope-Harman A, Nunley DR, Moffatt-Bruce S, Ross P, Martin SI. Multidrug-resistant Acinetobacter baumannii pneumonia in lung transplant recipients. J Heart Lung Transplant. 2008;27(7):804–7.
Lagana P, Melcarne L, Delia S. Acinetobacter baumannii and endocarditis, rare complication but important clinical relevance. Int J Cardiol. 2015;187:678–9.
Lenhard JR, Thamlikitkul V, Silveira FP, Garonzik SM, Tao X, Forrest A, et al. Polymyxin-resistant, carbapenem-resistant Acinetobacter baumannii is eradicated by a triple combination of agents that lack individual activity. J Antimicrob Chemother. 2017;72(5):1415–20.
Menegucci TC, Fedrigo NH, Lodi FG, Albiero J, Nishiyama SAB, Mazucheli J, et al. Pharmacodynamic effects of sulbactam/meropenem/polymyxin-B combination against extremely drug resistant Acinetobacter baumannii using checkerboard information. Microb Drug Resist. 2019;25(9):1266–74.
Ooi MH, Ngu SJ, Chor YK, Li J, Landersdorfer CB, Nation RL. Population pharmacokinetics of intravenous colistin in pediatric patients: implications for the selection of dosage regimens. Clin Infect Dis. 2019;69(11):1962–8.
Blumer JL, Reed MD, Kearns GL, Jacobs RF, Gooch WM 3rd, Yogev R, et al. Sequential, single-dose pharmacokinetic evaluation of meropenem in hospitalized infants and children. Antimicrob Agents Chemother. 1995;39(8):1721–5.
Schaad UB, Guenin K, Straehl P. Single-dose pharmacokinetics of intravenous sulbactam in pediatric patients. Rev Infect Dis. 1986;8(Suppl. 5):S512–7.
US FDA. Coly-Mycin® M parenteral colistimethate for injection, USP. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050108s033lbl.pdf. Accessed 29 Jul 2022.
US FDA. Merrem® IV (meropenem for injection), for intravenous use. 1996. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050706s037lbl.pdf. Accessed 29 Jul 2022.
Nahata MC, Vashi VI, Swanson RN, Messig MA, Chung M. Pharmacokinetics of ampicillin and sulbactam in pediatric patients. Antimicrob Agents Chemother. 1999;43(5):1225–9.
Jones H, Rowland-Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacomet Syst Pharmacol. 2013;14(2): e63.
Sy SK, Wang X, Derendorf H. Introduction to pharmacometrics and quantitative pharmacology with an emphasis on physiologically based pharmacokinetics. In: Derendorf H, Schmidt S, editors. Applied pharmacometrics. New York: Springer; 2014. p. 1–64.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Kitzis MD, Acar JF, Gutmann L. Antibacterial activity of meropenem against Gram-negative bacteria with a permeability defect and against staphylococci. J Antimicrob Chemother. 1989;24(Suppl. A):125–32.
Park SW, We JS, Kim GW, Choi SH, Park HS. Stability of new carbapenem DA-1131 to renal dipeptidase (dehydropeptidase I). Antimicrob Agents Chemother. 2002;46(2):575–7.
Ikawa K, Nakashima A, Morikawa N, Ikeda K, Murakami Y, Ohge H, et al. Clinical pharmacokinetics of meropenem and biapenem in bile and dosing considerations for biliary tract infections based on site-specific pharmacodynamic target attainment. Antimicrob Agents Chemother. 2011;55(12):5609–15.
Ganguly S, Edginton AN, Gerhart JG, Cohen-Wolkowiez M, Greenberg RG, Gonzalez D, et al. Physiologically based pharmacokinetic modeling of meropenem in preterm and term infants. Clin Pharmacokinet. 2021;60(12):1591–604.
Bax RP, Bastain W, Featherstone A, Wilkinson DM, Hutchison M, Haworth SJ. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother. 1989;24(Suppl. A):311–20.
Fawaz S, Barton S, Whitney L, Swinden J, Nabhani-Gebara S. Stability of meropenem after reconstitution for administration by prolonged infusion. Hosp Pharm. 2019;54(3):190–6.
Nakwan N, Usaha S, Chokephaibulkit K, Villani P, Regazzi M, Imberti R. Pharmacokinetics of colistin following a single dose of intravenous colistimethate sodium in critically ill neonates. Pediatr Infect Dis J. 2016;35(11):1211–4.
Michalopoulos AS, Falagas ME. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care. 2011;1(1):30.
Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40(9):1333–41.
Honore PM, Jacobs R, Joannes-Boyau O, Lochy S, Boer W, De Waele E, et al. Continuous renal replacement therapy-related strategies to avoid colistin toxicity: a clinically orientated review. Blood Purif. 2014;37(4):291–5.
Ma Z, Wang J, Nation RL, Li J, Turnidge JD, Coulthard K, et al. Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob Agents Chemother. 2009;53(7):2857–64.
Bouchene S, Marchand S, Couet W, Friberg LE, Gobin P, Lamarche I, et al. A whole-body physiologically based pharmacokinetic model for colistin and colistin methanesulfonate in rat. Basic Clin Pharmacol Toxicol. 2018;123(4):407–22.
Viel A, Henri J, Bouchene S, Laroche J, Rolland JG, Manceau J, et al. A population WB-PBPK model of colistin and its prodrug CMS in pigs: focus on the renal distribution and excretion. Pharm Res. 2018;35(5):92.
Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother. 2009;53(8):3430–6.
Sharma VD, Singla A, Chaudhary M, Taneja M. Population pharmacokinetics of fixed dose combination of ceftriaxone and sulbactam in healthy and infected subjects. AAPS PharmSciTech. 2016;17(5):1192–203.
Jaruratanasirikul S, Wongpoowarak W, Wattanavijitkul T, Sukarnjanaset W, Samaeng M, Nawakitrangsan M, et al. Population pharmacokinetics and pharmacodynamics modeling to optimize dosage regimens of sulbactam in critically ill patients with severe sepsis caused by Acinetobacter baumannii. Antimicrob Agents Chemother. 2016;60(12):7236–44.
Foulds G, Stankewich JP, Marshall DC, O’Brien MM, Hayes SL, Weidler DJ, et al. Pharmacokinetics of sulbactam in humans. Antimicrob Agents Chemother. 1983;23(5):692–9.
Biesdorf C, Martins FS, Sy SKB, Diniz A. Physiologically-based pharmacokinetics of ziprasidone in pregnant women. Br J Clin Pharmacol. 2019;85(5):914–23.
Martins FS, Zhu P, Heinrichs MT, Sy SKB. Physiologically based pharmacokinetic-pharmacodynamic evaluation of meropenem plus fosfomycin in paediatrics. Br J Clin Pharmacol. 2020;87(3):1012–23.
Sy SK, Asin-Prieto E, Derendorf H, Samara E. Predicting pediatric age-matched weight and body mass index. AAPS J. 2014;16(6):1372–9.
Valentin J. Basic anatomical and physiological data for use in radiological protection: reference values: ICRP publication 89. Ann ICRP. 2002;32(3–4):1–277.
Ince I, Solodenko J, Frechen S, Dallmann A, Niederalt C, Schlender J, et al. Predictive pediatric modeling and simulation using ontogeny information. J Clin Pharmacol. 2019;59(Suppl. 1):S95-103.
Open Systems Pharmacology. PK-Sim® Ontogeny Database, version 7.3. https://github.com/Open-Systems-Pharmacology/OSPSuite.Documentation/blob/master/PK-Sim Ontogeny Database Version 7.3.pdf. Accessed 28 May 2022.
McNamara PJ, Alcorn J. Protein binding predictions in infants. AAPS PharmSci. 2002;4(1):E4.
Wacharachaisurapol N, Sukkummee W, Anunsittichai O, Srisan P, Sangkhamal S, Chantharit P, et al. Dose recommendations for intravenous colistin in pediatric patients from a prospective, multicenter, population pharmacokinetic study. Int J Infect Dis. 2021;109:230–7.
Jansson B, Karvanen M, Cars O, Plachouras D, Friberg LE. Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J Pharm Biomed Anal. 2009;49(3):760–7.
Heffernan AJ, Roberts JA. Dose optimisation of antibiotics used for meningitis. Curr Opin Infect Dis. 2021;34(6):581–90.
Yokoyama Y, Matsumoto K, Ikawa K, Watanabe E, Morikawa N, Takeda Y. Population pharmacokinetic-pharmacodynamic target attainment analysis of sulbactam in patients with impaired renal function: dosing considerations for Acinetobacter baumannii infections. J Infect Chemother. 2015;21(4):284–9.
Delattre IK, Taccone FS, Jacobs F, Hites M, Dugernier T, Spapen H, et al. Optimizing beta-lactams treatment in critically-ill patients using pharmacokinetics/pharmacodynamics targets: are first conventional doses effective? Expert Rev Anti Infect Ther. 2017;15(7):677–88.
Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother. 2015;70(12):3291–7.
Tomaselli F, Maier A, Matzi V, Smolle-Juttner FM, Dittrich P. Penetration of meropenem into pneumonic human lung tissue as measured by in vivo microdialysis. Antimicrob Agents Chemother. 2004;48(6):2228–32.
Maglio D, Teng R, Thyrum PT, Nightingale CH, Nicolau DP. Pharmacokinetic profile of meropenem, administered at 500 milligrams every 8 hours, in plasma and cantharidin-induced skin blister fluid. Antimicrob Agents Chemother. 2003;47(5):1771–3.
Markantonis SL, Markou N, Fousteri M, Sakellaridis N, Karatzas S, Alamanos I, et al. Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother. 2009;53(11):4907–10.
Rodvold KA, Gotfried MH, Isaacs RD, O’Donnell JP, Stone E. Plasma and intrapulmonary concentrations of ETX2514 and sulbactam following Intravenous administration of ETX2514SUL to healthy adult subjects. Antimicrob Agents Chemother. 2018;62(11):e01089-e1118.
Newsom SW, Palsingh J, Wells FC, Kelly HC. Penetration of meropenem into heart valve tissue. J Antimicrob Chemother. 1995;36(Suppl. A):57–62.
Shetty AK, Zanirati G. The interstitial system of the brain in health and disease. Aging Dis. 2020;11(1):200–11.
Aoki N, Tateda K, Kikuchi Y, Kimura S, Miyazaki C, Ishii Y, et al. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J Antimicrob Chemother. 2009;63(3):534–42.
Albiero J, Mazucheli J, Barros J, Szczerepa M, Nishiyama SAB, Carrara-Marroni FE, et al. Pharmacodynamic attainment of the synergism of meropenem and fosfomycin combination against Pseudomonas aeruginosa producing metallo-beta-lactamase. Antimicrob Agents Chemother. 2019;63(6):e00126-e219.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 2020. www.clsi.org. Accessed 29 Jul 2022.
Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39.
Huang JX, Blaskovich MA, Pelingon R, Ramu S, Kavanagh A, Elliott AG, et al. Mucin binding reduces colistin antimicrobial activity. Antimicrob Agents Chemother. 2015;59(10):5925–31.
Abodakpi H, Gohlke J, Chang KT, Chow DS, Tam VH. Analytical and functional determination of polymyxin B protein binding in serum. Antimicrob Agents Chemother. 2015;59(11):7121–3.
Yokoyama Y, Matsumoto K, Ikawa K, Watanabe E, Shigemi A, Umezaki Y, et al. Pharmacokinetic/pharmacodynamic evaluation of sulbactam against Acinetobacter baumannii in in vitro and murine thigh and lung infection models. Int J Antimicrob Agents. 2014;43(6):547–52.
Fish DN. Meropenem in the treatment of complicated skin and soft tissue infections. Ther Clin Risk Manag. 2006;2(4):401–15.
Zobell JT, Young DC, Waters CD, Ampofo K, Cash J, Marshall BC, et al. A survey of the utilization of anti-pseudomonal beta-lactam therapy in cystic fibrosis patients. Pediatr Pulmonol. 2011;46(10):987–90.
Kanra G. Experience with ampicillin/sulbactam in severe infections. J Int Med Res. 2002;30(Suppl. 1):20A-30A.
Fotakopoulos G, Makris D, Chatzi M, Tsimitrea E, Zakynthinos E, Fountas K. Outcomes in meningitis/ventriculitis treated with intravenous or intraventricular plus intravenous colistin. Acta Neurochir. 2016;158(3):603–10 (discussion 10).
Florescu DF, Qiu F, McCartan MA, Mindru C, Fey PD, Kalil AC. What is the efficacy and safety of colistin for the treatment of ventilator-associated pneumonia? A systematic review and meta-regression. Clin Infect Dis. 2012;54(5):670–80.
Bian X, Liu X, Feng M, Bergen PJ, Li J, Chen Y, et al. Enhanced bacterial killing with colistin/sulbactam combination against carbapenem-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2021;57(2): 106271.
Shi H, Lee JS, Park SY, Ko Y, Eom JS. Colistin plus carbapenem versus colistin monotherapy in the treatment of carbapenem-resistant Acinetobacter baumannii pneumonia. Infect Drug Resist. 2019;12:3925–34.
Maifiah MH, Creek DJ, Nation RL, Forrest A, Tsuji BT, Velkov T, et al. Untargeted metabolomics analysis reveals key pathways responsible for the synergistic killing of colistin and doripenem combination against Acinetobacter baumannii. Sci Rep. 2017;30(7):45527.
Pettit RS, Neu N, Cies JJ, Lapin C, Muhlebach MS, Novak KJ, et al. Population pharmacokinetics of meropenem administered as a prolonged infusion in children with cystic fibrosis. J Antimicrob Chemother. 2016;71(1):189–95.
Iosifidis E, Antachopoulos C, Ioannidou M, Mitroudi M, Sdougka M, Drossou-Agakidou V, et al. Colistin administration to pediatric and neonatal patients. Eur J Pediatr. 2010;169(7):867–74.
Goverman J, Weber JM, Keaney TJ, Sheridan RL. Intravenous colistin for the treatment of multi-drug resistant, gram-negative infection in the pediatric burn population. J Burn Care Res. 2007;28(3):421–6.
Lee CH, Tang YF, Su LH, Chien CC, Liu JW. Antimicrobial effects of varied combinations of meropenem, sulbactam, and colistin on a multidrug-resistant Acinetobacter baumannii isolate that caused meningitis and bacteremia. Microb Drug Resist. 2008;14(3):233–7.
Fan B, Guan J, Wang X, Cong Y. Activity of colistin in combination with meropenem, tigecycline, fosfomycin, fusidic acid, rifampin or sulbactam against extensively drug-resistant Acinetobacter baumannii in a murine thigh-infection model. PLoS One. 2016;11(6): e0157757.
Liu X, Zhao M, Chen Y, Bian X, Li Y, Shi J, et al. Synergistic killing by meropenem and colistin combination of carbapenem-resistant Acinetobacter baumannii isolates from Chinese patients in an in vitro pharmacokinetic/pharmacodynamic model. Int J Antimicrob Agents. 2016;48(5):559–63.
Saelim W, Changpradub D, Thunyaharn S, Juntanawiwat P, Nulsopapon P, Santimaleeworagun W. Colistin plus sulbactam or fosfomycin against carbapenem-resistant Acinetobacter baumannii: improved efficacy or decreased risk of nephrotoxicity? Infect Chemother. 2021;53(1):128–40.
Lim SMS, Heffernan AJ, Zowawi HM, Roberts JA, Sime FB. Semi-mechanistic PK/PD modelling of meropenem and sulbactam combination against carbapenem-resistant strains of Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis. 2021;40(9):1943–52.
Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400.
Rayner CR, Smith PF, Andes D, Andrews K, Derendorf H, Friberg LE, et al. Model-informed drug development for anti-Iifectives: state of the art and future. Clin Pharmacol Ther. 2021;109(4):867–91.
Thorsted A, Bouchene S, Tano E, Castegren M, Lipcsey M, Sjolin J, et al. A non-linear mixed effect model for innate immune response: In vivo kinetics of endotoxin and its induction of the cytokines tumor necrosis factor alpha and interleukin-6. PLoS ONE. 2019;14(2): e0211981.
Couet W, Gregoire N, Marchand S, Mimoz O. Colistin pharmacokinetics: the fog is lifting. Clin Microbiol Infect. 2012;18(1):30–9.
Martins FS, Zhu P, Heinrichs MT, Sy SKB. Physiologically based pharmacokinetic-pharmacodynamic evaluation of meropenem plus fosfomycin in paediatrics. Br J Clin Pharmacol. 2021;87(3):1012–23.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by a grant from Shandong Provincial Natural Science Foundation (ZR2019BC025).
Conflict of interest
CO is the owner of SunLife Biopharma, which is a private consulting firm. All other authors have nothing to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors (1) contributed to the design of the study, acquisition, or analysis of data, (2) drafted or revised the article for intellectual content, and (3) approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, S., Zhang, J., Lv, Z. et al. Prediction of Tissue Exposures of Meropenem, Colistin, and Sulbactam in Pediatrics Using Physiologically Based Pharmacokinetic Modeling. Clin Pharmacokinet 61, 1427–1441 (2022). https://doi.org/10.1007/s40262-022-01161-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01161-y