Abstract
The production of autologous T cells expressing a chimaeric antigen receptor (CAR) is time-consuming, costly and occasionally unsuccessful. T-cell-derived induced pluripotent stem cells (TiPS) are a promising source for the generation of ‘off-the-shelf’ CAR T cells, but the in vitro differentiation of TiPS often yields T cells with suboptimal features. Here we show that the premature expression of the T-cell receptor (TCR) or a constitutively expressed CAR in TiPS promotes the acquisition of an innate phenotype, which can be averted by disabling the TCR and relying on the CAR to drive differentiation. Delaying CAR expression and calibrating its signalling strength in TiPS enabled the generation of human TCR– CD8αβ+ CAR T cells that perform similarly to CD8αβ+ CAR T cells from peripheral blood, achieving effective tumour control on systemic administration in a mouse model of leukaemia and without causing graft-versus-host disease. Driving T-cell maturation in TiPS in the absence of a TCR by taking advantage of a CAR may facilitate the large-scale development of potent allogeneic CD8αβ+ T cells for a broad range of immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA-sequencing data are available from the Gene Expression Omnibus under accession number GSE210364. Source data for tumour growth are provided with this paper. The raw and analysed datasets generated during the study are available from the corresponding author on reasonable request.
Change history
12 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41551-024-01181-y
References
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Sadelain, M., Rivière, I. & Riddell, S. Therapeutic T cell engineering. Nature 545, 423–431 (2017).
Globerson Levin, A., Rivière, I., Eshhar, Z. & Sadelain, M. CAR T cells: building on the CD19 paradigm. Eur. J. Immunol. 51, 2151–2163 (2021).
Maldini, C. R., Ellis, G. I. & Riley, J. L. CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol. 18, 605–616 (2018).
Chen, Y., Sun, J., Liu, H., Yin, G. & Xie, Q. Immunotherapy deriving from CAR-T cell treatment in autoimmune diseases. J. Immunol. Res. 2019, 5727516 (2019).
Wang, X. & Rivière, I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol. Ther. Oncolytics 3, 16015 (2016).
Allen, E. S. et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion 57, 1133–1141 (2017).
Themeli, M., Rivière, I. & Sadelain, M. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell 16, 357–366 (2015).
Depil, S., Duchateau, P., Grupp, S. A., Mufti, G. & Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug. Discov. 19, 185–199 (2020).
Benjamin, R. et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet 396, 1885–1894 (2020).
Qasim, W. et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 9, eaaj2013 (2017).
Themeli, M. et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 31, 928–933 (2013).
Wang, B. et al. Generation of hypoimmunogenic T cells from genetically engineered allogeneic human induced pluripotent stem cells. Nat. Biomed. Eng. 5, 429–440 (2021).
Woan, K. V. et al. Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell 28, 2062–2075.e5 (2021).
Harada, S. et al. Dual-antigen targeted iPSC-derived chimeric antigen receptor-T cell therapy for refractory lymphoma. Mol. Ther. 30, 534–549 (2022).
Ueda, T. et al. Non-clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti-glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci. 111, 1478–1490 (2020).
Yui, M. A. & Rothenberg, E. V. Developmental gene networks: a triathlon on the course to T cell identity. Nat. Rev. Immunol. 14, 529–545 (2014).
Pardoll, D. M. et al. Thymus-dependent and thymus-independent developmental pathways for peripheral T cell receptor-gamma delta-bearing lymphocytes. J. Immunol. 140, 4091–4096 (1988).
Fehling, H. J., Krotkova, A., Saint-Ruf, C. & von Boehmer, H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature 375, 795–798 (1995).
Hogquist, K. A., Gavin, M. A. & Bevan, M. J. Positive selection of CD8+ T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J. Exp. Med. 177, 1469–1473 (1993).
Baldwin, T. A., Sandau, M. M., Jameson, S. C. & Hogquist, K. A. The timing of TCR alpha expression critically influences T cell development and selection. J. Exp. Med. 202, 111–121 (2005).
Washburn, T. et al. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell 88, 833–843 (1997).
Mohtashami, M. et al. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J. Immunol. 185, 867–876 (2010).
Van de Walle, I. et al. Specific Notch receptor-ligand interactions control human TCR-αβ/γδ development by inducing differential Notch signal strength. J. Exp. Med. 210, 683–697 (2013).
Ramello, M.C. et al. An immunoproteomic approach to characterize the CAR interactome and signalosome. Sci. Signal. 12, eaap9777 (2019).
Maluski, M. et al. Chimeric antigen receptor-induced BCL11B suppression propagates NK-like cell development. J. Clin. Invest. 129, 5108–5122 (2019).
Feucht, J. et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 25, 82–88 (2019).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Res, P., Blom, B., Hori, T., Weijer, K. & Spits, H. Downregulation of CD1 marks acquisition of functional maturation of human thymocytes and defines a control point in late stages of human T cell development. J. Exp. Med. 185, 141–151 (1997).
Haynes, B. F., Singer, K. H., Denning, S. M. & Martin, M. E. Analysis of expression of CD2, CD3, and T cell antigen receptor molecules during early human fetal thymic development. J. Immunol. 141, 3776–3784 (1988).
Fujii, Y., Okumura, M., Inada, K., Nakahara, K. & Matsuda, H. CD45 isoform expression during T cell development in the thymus. Eur. J. Immunol. 22, 1843–1850 (1992).
Van de Walle, I. et al. An early decrease in Notch activation is required for human TCR-alphabeta lineage differentiation at the expense of TCR-gammadelta T cells. Blood 113, 2988–2998 (2009).
Yashiro-Ohtani, Y. et al. Pre-TCR signaling inactivates Notch1 transcription by antagonizing E2A. Genes Dev. 23, 1665–1676 (2009).
Hernández-Hoyos, G., Anderson, M. K., Wang, C., Rothenberg, E. V. & Alberola-Ila, J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity 19, 83–94 (2003).
Dolens, A. C. et al. Distinct Notch1 and BCL11B requirements mediate human γδ/αβ T cell development. EMBO Rep. 21, e49006 (2020).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9, 279–286 (2003).
Zhao, Z. et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28, 415–428 (2015).
Ciofani, M., Knowles, G. C., Wiest, D. L., von Boehmer, H. & Zúñiga-Pflücker, J. C. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity 25, 105–116 (2006).
Hayes, S. M., Li, L. & Love, P. E. TCR signal strength influences alphabeta/gammadelta lineage fate. Immunity 22, 583–593 (2005).
Groettrup, M. et al. A novel disulfide-linked heterodimer on pre-T cells consists of the T cell receptor beta chain and a 33 kd glycoprotein. Cell 75, 283–294 (1993).
Borowski, C., Li, X., Aifantis, I., Gounari, F. & von Boehmer, H. Pre-TCRalpha and TCRalpha are not interchangeable partners of TCRbeta during T lymphocyte development. J. Exp. Med. 199, 607–615 (2004).
Terrence, K., Pavlovich, C. P., Matechak, E. O. & Fowlkes, B. J. Premature expression of T cell receptor (TCR)alphabeta suppresses TCRgammadelta gene rearrangement but permits development of gammadelta lineage T cells. J. Exp. Med. 192, 537–548 (2000).
Reizis, B. & Leder, P. Direct induction of T lymphocyte-specific gene expression by the mammalian Notch signaling pathway. Genes Dev. 16, 295–300 (2002).
Haks, M. C. et al. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity 22, 595–606 (2005).
Kim, Y. M. et al. Expression of 4-1BB and 4-1BBL in thymocytes during thymus regeneration. Exp. Mol. Med. 41, 896–911 (2009).
Groves, T., Parsons, M., Miyamoto, N. G. & Guidos, C. J. TCR engagement of CD4+CD8+ thymocytes in vitro induces early aspects of positive selection, but not apoptosis. J. Immunol. 158, 65–75 (1997).
Anderson, G., Hare, K. J. & Jenkinson, E. J. Positive selection of thymocytes: the long and winding road. Immunol. Today 20, 463–468 (1999).
McFarland, R. D., Douek, D. C., Koup, R. A. & Picker, L. J. Identification of a human recent thymic emigrant phenotype. Proc. Natl Acad. Sci. USA 97, 4215–4220 (2000).
Iriguchi, S. et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat. Commun. 12, 430 (2021).
Ito, T. et al. The therapeutic potential of multiclonal tumoricidal T cells derived from tumor infiltrating lymphocyte-1derived iPS cells. Commun. Biol. 4, 694 (2021).
Valamehr, B. et al. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Rep. 2, 366–381 (2014).
Nagano, S. et al. High frequency production of T cell-derived iPSC clones capable of generating potent cytotoxic T cells. Mol. Ther. Methods Clin. Dev. 16, 126–135 (2020).
Fraietta, J. A. et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 558, 307–312 (2018).
Poirot, L. et al. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res. 75, 3853–3864 (2015).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, 1001 (2020).
Ruella, M. et al. A cellular antidote to specifically deplete anti-CD19 chimeric antigen receptor-positive cells. Blood 135, 505–509 (2020).
Mansilla-Soto, J. et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat. Med. 28, 345–352 (2022).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Rivière, I., Brose, K. & Mulligan, R. C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc. Natl Acad. Sci. USA 92, 6733–6737 (1995).
Hamieh, M. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019).
Cichocki, F. et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci. Transl. Med. 12, eaaz5618 (2020).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Valamehr, B. et al. A novel platform to enable the high-throughput derivation and characterization of feeder-free human iPSCs. Sci. Rep. 2, 213 (2012).
Maher, J., Brentjens, R. J., Gunset, G., Rivière, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Gong, M. C. et al. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia 1, 123–127 (1999).
Acknowledgements
We thank G. Gunset for logistical and technical assistance, M. Sættersmoen for advice on NK-cell culture, A. Iyer for support with statistical analyses and E. Ortiz for support in cell culture. We thank the SKI Cell Therapy and Cell Engineering Facility, the Flow Cytometry core facility, Integrated Genomics Operation, Antitumor Assessment and Animal Core Facilities for their expert assistance. We also thank Y.-S. Lai, C.-W. Chang, A. Witty, B.-H. Yang, M. Ribadi, J. Huffman, H. Shaked, R. Bjordahl and B. Whitlock (Fate Therapeutics Inc.) for technical contributions. This work was supported by the Tri-I Stem Cell Initiative, the Tow Foundation, Cycle for Survival, the Marie-Josée and Henry R. Kravis Center for Molecular Oncology, Fate Therapeutics Inc., and NCI grant P30 CA08748. S.J.C.v.d.S. and M.T. were supported by a New York Stem Cell Foundation Druckenmiller Fellowship.
Author information
Authors and Affiliations
Contributions
S.J.C.v.d.S. designed the study, performed experiments, analysed and interpreted data, and wrote the manuscript. P.L.L. and R.M.P. performed experiments, analysed and interpreted data. H.X. performed RNA-sequencing analysis. M.P.D., V.A., Y.S., M.H., J.M.-S., J.E. and A.C. performed experiments. X.W. and I.R. generated and provided clinical experimental materials. R.A., T.L., R.C. and B.V. generated TiPS lines, developed iCD34 methodology. M.T. and I.R. contributed to experimental design and data analysis. M.S. designed the study, analysed and interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.A., T.L., R.C. and B.V. are employees of Fate Therapeutics Inc. and have equity in the company. M.S. reports research funding from Takeda Pharmaceuticals, Atara Biotherapeutics and Fate Therapeutics. M.S. served on the scientific advisory board of St Jude Children’s Research Hospital. Memorial Sloan Kettering has licensed intellectual property on which M.S. is a named inventor to Fate Therapeutics. M.S. does not receive consultation fees or forms of remuneration. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Laurent Poirot, Axel Schambach and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 T lymphoid commitment of hES, FiPS and TiPS on OP9-mDLL1.
a, Flow cytometric analysis of pluripotency marker expression on H1, FiPS and WT-TiPS b, Flow cytometric analysis of T lymphoid markers of H1, FiPS and WT-TiPS during differentiation on OP9-mDLL1 at indicated timepoints. Plots depicting CD7/CD5 are gated on live CD45+ cells, plots depicting CD3/TCRαβ, CD4/CD8α and CD8α/CD8β are gated on live CD45+CD7+ cells. CD3/TCRαβ and CD4/CD8α at D40 are as presented in Fig. 1b.
Extended Data Fig. 2 Generation, validation, and differentiation of TRAC–/–-TiPS.
a, CRISPR/Cas9-targeted integration of EF1a-GFP-P2A-Puromycing-bGHpA (G2AP) expression unit into the TRAC locus. Top, TRAC locus; middle, plasmid containing the G2AP expression unit flanked by homology arms; bottom, edited TRAC locus. ‘FWD’ and ‘REV’ indicate the location of the forward and reverse primers used in b. b, PCR validation of G2AP integration into the TRAC locus of TiPS clones. c, Flow cytometric analysis of pluripotency marker expression on TRAC–/–-TiPS. Gated on live cells. d, T lymphoid makers of WT-TiPS and TRAC–/–-TiPS during differentiation on OP9-mDLL1 at the indicated timepoints. Gated on live CD45+ cells. D40 is as presented in Fig. 1c.
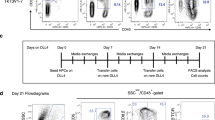
Extended Data Fig. 3 Early T lymphoid commitment of WT-TiPS and CAR−TiPS on human Notch ligands.
a, SFG γRV plasmid design to transduce human Notch ligands (DLL1, DLL4, JAG1 or JAG2) into parental OP9 cells. b, Notch ligand expression on engineered OP9 lines. Filled grey histogram corresponds to stained parental OP9 cells, and open black histogram to transduced OP9 cells. c, DTX1 induction in WT-TiPS by OP9 expressing indicated Notch ligand. D20 differentiating WT-TiPS cells were co-cultured with indicated OP9. DTX1 induction was measured by ddPCR, relative to endogenous RPL13A. The fold change was calculated relative to 0 h. Data shown is average of n = 2 technical replicates. d, g, Flow cytometric analysis of T lymphoid commitment marker expression (CD7, CD5, TCRαβ and CD56) of WT-TiPS (d) and CAR−TiPS (g) differentiated on OP9 expressing indicated human Notch ligands. Gated on live CD45+ cells. e, Flow cytometric analysis of pluripotency marker expression on CAR-TiPS. Gated on live cells. f, Phosphorylated-ERK1/2 levels in WT-TiPS (blue) and CAR-TiPS (red) on D35 (n = 3 technical replicates, p = 0.0014). h, Phenotype (left panels) and apoptosis levels (right panels) of WT-TiPS (top) and CAR-TiPS (bottom) from D27 – D35 of differentiation on OP9-DLL4. Percentage of apoptotic cells in each T lineage developmental stage was based on percentage live Annexin-V+ cells. * P < 0.05, ** P < 0.01, *** P < 0.001, Welch’s 2-sample two-sided t test, data are means ± s.d (f).
Extended Data Fig. 4 CD8αβ single positive CAR+ iT cell development.
WT-TiPS were differentiated on OP9-DLL4 and transduced to express the 1928z CAR at D35 utilizing γRV SFG-1928z-P2A-LNGFR. Cells were expanded for 7 days in expansion media supplemented with IL-2. a, CD4/CD8αβ expression prior to transduction (D35) and on D42 in LNGFR+ cells, LNGFR- cells and untransduced control cells which remained in differentiation on OP9-DLL4. Gated on live CD45+ cells. b, Cytotoxic activity of CAR+ iT cells in a 18 h bioluminescence assay, using FFLuc-NALM6 as target cells (n = 3 technical replicates, data are mean ± s.d). c, CRISPR/Cas9-targeted integration of CAR transgene into the TRAC locus. Top, TRAC locus; middle, plasmid containing the CAR transgene cassette flanked by homology arms; bottom, edited TRAC locus. d, f, PCR validation of CAR integration into the TRAC locus of TRAC-1928z-TiPS (d) and TRAC-1XX-TiPS (f) clones. e, g, Pluripotency marker expression on TRAC-1928z-TiPS (e) and TRAC-1XX-TiPS (g), gated on live cells.
Extended Data Fig. 5 T lineage commitment of TRAC-CAR-TiPS.
a, T lineage commitment marker expression (CD7/CD5, CD4/CD8α, CD8α/CD8β) of WT-TiPS (left), TRAC-1928z-TiPS (middle) and TRAC-1XX-TiPS (right) on OP9-DLL4 at the indicated timepoints. CD7/CD5 is gated on live CD45+ cells, others are gated on live CD45+CD7+ cells. b, Flow cytometric analysis of T cell phenotype markers of D35 DP TRAC-1XX-iT cells. Gated on live CD45+CD7+CD4+CD8αβ+ cells. c, Intracellular and cell-surface expression of CD3 and TCRαβ on D35 TRAC-1XX-iT cells.
Extended Data Fig. 6 Tonic ITAM phosphorylation in CAR+ T cells.
a, Representative flow cytometry plot of CAR expression and pITAM1 (top panel) or pITAM3 (bottom panel) in PBMC-derived T cells expressing γRV-1928z, TRAC-1928z or TRAC-1XX (gated on live CAR+), or in control TRAC–/– cells (gated on live CAR-). b, c, Percentage of pITAM1+ (b) and pITAM3+ (c) in the populations shown in a (n = 4–5 biological replicates, data are means ± s.d.).
Extended Data Fig. 7 DP TRAC-1XX-iT cell mature to CD8αβ SP iT cells on 3T3-CD19-41BBL.
a, c, Flow cytometric analysis of D42 cells matured on 3T3-CD19 (a) or 3T3-CD19-41BBL (c). Gated on live CD45+CD7+ cells. b, Flow cytometric analysis of D35 and D42 phenotypes of stimulated DP TRAC-1XX-iT cells. D35 TRAC-1XX-iT cells were sorted for a CD4+CD8αβ+ DP phenotype, stimulated on 3T3-CD19-41BBL and expanded for seven days. Gated on live CD45+CD7+ cells. d, Fold Expansion and T cell phenotype marker expression of TRAC-1XX-iT cells matured on 3T3-CD19-41BBL (3T3) or recombinant CD19-Fc. e, 4 h cytotoxicity assay of 3T3-CD19-41BBL stimulated TRAC-1XX-iT cells in response to NALM6-CD19+ (red) an NALM6-CD19–/– (blue) as target cells (n = 3 technical replicates, data are means ± s.d.) f, CD19 expression on primary CLL cells. Filled grey histogram are unstained CLL cells, open red histogram are stained CLL cells.
Extended Data Fig. 8 Comparison of CD8αβ TRAC-1XX-iT cells and peripheral blood lymphocytes.
a, Representative examples of lymphoid phenotype marker expression in CD8αβ TRAC-1XX-iT (CD8αβ iT, red), CD8αβ αβTCR-T (CD8, blue), CD4 αβTCR-T (CD4, orange), γδTCR-T (γδ, green) and NK cells (NK, purple). CD8αβ TRAC-1XX-iT cells are the same as represented in Fig. 5a. b, Variability of lymphoid phenotype marker expression in CD8αβ TRAC-1XX-iT cells (n = 3-4 biological replicates, data are means ± s.d). Biological replicates shown are samples utilized in RNA analysis (Fig. 5b,c). c, Principal Component Analysis comparing TRAC-1XX CD8αβ αβTCR-T cells (CD8, n = 4), TRAC-1XX CD4 αβTCR-T cells (CD4, n = 3), γRV-1XX γδTCR-T cells (γδ, n = 4), γRV-1XX NK cells (NK, n = 4) and CD8αβ+ TRAC-1XX-iT cells (iT CD8αβ, n = 4).
Extended Data Fig. 9 Functional comparison of TRAC-1XX-iT, CAR-iT and CD8 TRAC-1XX.
Functional comparison of healthy-donor peripheral blood TRAC-1XX CD8αβ αβTCR-T (CD8 TRAC-1XX), CAR-iT and TRAC-1XX-iT cells. CD8 TRAC-1XX cell doses represent number of CAR+ cells utilized in the assay. a, CAR and CD3 expression in CD8 TRAC-1XX, CAR-iT and TRAC-1XX-iT cells (black line) compared to unstained control (grey filled histogram). b, 18-h Incucyte cytotoxicity assay with NLR+ CD19–/– NALM6 target cells (n = 3 technical replicates). c, 4 h intracellular cytokine detection in T cells stimulated with NALM6 CD19+ target cells (at a 1:1 E:T), PMA/Ionomycin, NALM6 CD19–/– target cells (at a 1:1 E:T) unstimulated controls (n = 3 technical replicates). d, Twenty-four h cytokine secretion using NALM6-CD19–/– as target cells at a 1:1 E:T (n = 11-18 biological replicates, left panel) or unstimulated control (n = 11-18 biological replicates, right panel). e, Schematic representation of the NALM6 in vivo tumour model. f, Kaplan-Meier analysis of tumourfree survival (2×106 TRAC-1XX-iT vs 2×106 CD8 TRAC-1XX p = 0.0062, 2×106 TRAC-1XX-iT vs 1×105 CD8 TRAC-1XX p = 0.0034). * P < 0.05, ** P < 0.01, *** P < 0.001, Chi-Square test (b) log-rank Mantel-Cox test (f). All data are means ± s.d.
Extended Data Fig. 10 TRAC-1XX-iT function compared to healthy donor peripheral blood-derived CD8 TRAC-1XX T cells.
In vivo functional comparison of healthy-donor peripheral blood TRAC-1XX CD8αβ αβTCR-T (CD8 TRAC-1XX), TRAC-1XX-iT cells. CD8 TRAC-1XX cell doses represent number of CAR+ cells utilized in the assay. a, CAR and CD3 expression in CD8 TRAC-1XX and TRAC-1XX-iT cells (black line) compared to unstained control (grey filled histogram). b, Enumeration of tumour cells in the bone marrow and T cells in bone marrow, spleen and blood 6 days or 12 days after T-cell infusion (n = 2-3 mice, T cell in bone marrow day 12, CD8 TRAC-1XX vs TRAC-1XX-iT p = 0.0161, T cells in spleen day 12 CD8 TRAC-1XX vs TRAC-1XX-iT p = 0.0052). c, Phenotype of CD8 cells prior to infusion (day 0, n = 1) and of cells derived from the bone marrow on day 6 (n = 3 mice) and 12 (n = 3 mice). * P < 0.05, ** P < 0.01, *** P < 0.001 Welch’s 2-sample two-sided t test (b). All data are means ± s.d.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Source Data for Figs. 4, 6, 7 and Extended Data Fig. 9
In vivo tumour-growth data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van der Stegen, S.J.C., Lindenbergh, P.L., Petrovic, R.M. et al. Generation of T-cell-receptor-negative CD8αβ-positive CAR T cells from T-cell-derived induced pluripotent stem cells. Nat. Biomed. Eng 6, 1284–1297 (2022). https://doi.org/10.1038/s41551-022-00915-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00915-0
This article is cited by
-
CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn
Nature Reviews Clinical Oncology (2024)
-
Current approaches to develop “off-the-shelf” chimeric antigen receptor (CAR)-T cells for cancer treatment: a systematic review
Experimental Hematology & Oncology (2023)
-
Humanized mouse models for immuno-oncology research
Nature Reviews Clinical Oncology (2023)
-
Generation of antigen-specific mature T cells from RAG1−/−RAG2−/−B2M−/− stem cells by engineering their microenvironment
Nature Biomedical Engineering (2023)
-
“Off-the-Shelf” Allogeneic CAR Cell Therapy—Neglected HvG Effect
Current Treatment Options in Oncology (2023)