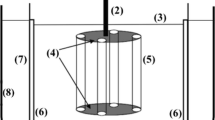
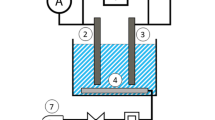
Abstract
The presence of high levels of heavy metals in wastewaters is known to pose a serious threat to the environment. Because of their numerous industrial, domestic, agricultural, medical, and technical applications, they are widely distributed in the environment, raising worries about their possible effects on human health and the environment. In this work, a cylindrical-expanded aluminum sheet placed in an agitated vessel was used to recover copper ions from synthetic wastewater. The influence of initial Cu2+ ion concentration, rotating speed, solution pH, aluminum mesh number, temperature, and the presence of different types of surfactants on the kinetics of the Cu+2 ions cementation was investigated. The current study found that the rate of copper cementation rises with increasing rotational speed, starting copper ion concentration, temperature, and the mesh number of the expanded aluminum cylindrical sheet, but the pH has no influence within range examined. Surfactants reduce the rate of cementation by a percentage ranging from 9.38 to 75.19 for cetyltrimethylammonium bromide (CTMAB) and 19.27 to 78.53 for sodium dodecyl sulfate (SDS) depending on mesh number and rpm. The mass transfer data of the present study have been correlated for by a dimensionless equation from which the rate of cementation can be estimated at various conditions within the range of the applicability of the equation.
Graphical Abstract














Similar content being viewed by others
Abbreviations
- A:
-
Surface area of the expanded aluminum cylinder, cm2
- Ao :
-
Arrhenius equation frequency factor, cm/s
- Q:
-
Solution volume, cm3
- Co :
-
Initial concentration of copper ions, mol/cm3
- C:
-
Concentration of copper ions at any time, mol/cm3
- d:
-
Diameter of the expanded aluminum cylinder, cm
- de :
-
Screen’s hydraulic diameter, cm
- dw :
-
Screen’s wire diameter, cm
- di :
-
Impeller diameter, cm
- D:
-
Diffusivity of copper ions, cm2/s
- eo :
-
Standard electrode potential, Volt
- Eo :
-
Standard potential of the cementation overall reaction, Volt
- E:
-
Activation Energy, kcal/mol
- F:
-
Faraday’s constant, 96,500 Coulumbs/mol
- k:
-
Mass transfer coefficient, cm/s
- K:
-
Volumetric mass transfer coefficient at the expanded aluminum cylinder, cm3/s
- \(\overline{K }\) :
-
Equilibrium constant, cm3/mol
- Ko :
-
Volumetric mass transfer coefficient in the presence of the surfactants, cm3/s
- Ks :
-
Volumetric mass transfer coefficient at the solid aluminum sheet, cm3/s
- R:
-
Universal gas constant, kcal/(mol K)
- t:
-
Reaction time, s
- T:
-
Absolute temperature, K
- Z:
-
Number of electrons involved in the cementation reaction
- \(\alpha \) :
-
Inhibition efficiency
- \(\Delta {G}_{o}\) :
-
Standard free energy, J/mol
- \(\varnothing \) :
-
Porosity
- \(\varepsilon \) :
-
Enhancement ratio
- \(\mu \) :
-
Solution viscosity, Poise
- \(\omega \) :
-
Impeller rotational speed, rpm
- \(\rho \) :
-
Solution density, g/cm3
- Re:
-
Impeller Reynolds number \(\left(\frac{\rho N {d}_{i}^{2}}{ \mu }\right)\)
- Sc:
-
Schmidt number \(\left(\frac{\mu }{\rho D}\right)\)
- Sh:
-
Sherwood number \(\left(\frac{K}{D d}\right)\)
References
Bhatti MS, Kapoor D, Kalia RK, Reddy AS, Thukral AK (2011) RSM and ANN modeling for electrocoagulation of copper from simulated wastewater: multi objective optimization using genetic algorithm approach. Desalination 274(1):74–80
Rengaraj S, Kim Y, Joo CK, Yi J (2004) Removal of copper from aqueous solution by aminated and protonated mesoporous aluminas: kinetics and equilibrium. J Colloid Interface Sci 273(1):14–21
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption-removal of copper. J Hazard Mater 80(1):33–42
Ibrahim BS, Abdel-Aziz MH, El-Ashtoukhy E-SZ, Zewail TM, Zatout AA, Sedahmed GH (2021) Cementation of copper on zinc in agitated vessels equipped with perforated baffles as turbulence promoters. Min Metall Exploration 38(2):1203–1213
Zaabar A, Aitout R, Amoura D, Maizia R, Makhloufi L, Saidani B (2019) Effect of nettle plant extract on the overconsumption diminution of zinc as sacrificial metal during cementation of copper. Miner Eng 142:105933
Shishkin A, Mironovs V, Vu H, Novak P, Baronins J, Polyakov A, Ozolins J (2018) Cavitation-dispersion method for copper cementation from wastewater by iron powder. Metals 8(11):920
Granata G, Tsendorj U, Liu W, Tokoro C (2019) Direct recovery of copper nanoparticles from leach pad drainage by surfactant-assisted cementation with iron powder. Colloids Surf A 580:123719
Demirkıran N, Özdemir GDT, Dardağan M (2020) Application of response surface method to copper cementation by metallic aluminum particles. Ch&ChT 14(4):590–596
Abdel-Aziz MH, El-Ashtoukhy E-SZ, Bassyouni M (2016) Recovery of copper from effluents by cementation on aluminum in a multirotating cylinder-agitated vessel. Metall and Mater Trans B 47(1):657–665
Annamalai V, Hiskey JB, Murr LE (1978) The effects of kinetic variables on the structure of copper deposits cemented on pure aluminum discs: a scanning electron microscopic study. Hydrometallurgy 3(2):163–180
Mackinnon DJ, Ingraham TR (1970) Kinetics of Cu (II) cementation on a pure aluminum disc in acidic sulphate solutions. Can Metall Q 9(3):443–448
Djokić SS (1996) Cementation of copper on aluminum in alkaline solutions. J Electrochem Soc 143(4):1300–1305
Dib A, Makhloufi L (2004) Cementation treatment of copper in wastewater: mass transfer in a fixed bed of iron spheres. Chem Eng Process 43(10):1265–1273
Gros F, Baup S, Aurousseau M (2008) Intensified recovery of copper in solution: cementation onto iron in fixed or fluidized bed under electromagnetic field. Chem Eng Process 47(3):295–302
Gros F, Baup S, Aurousseau M (2011) Copper cementation on zinc and iron mixtures: Part 2: fluidized bed configuration. Hydrometallurgy 106(1):119–126
Mubarak AA, El-Shazly AH, Konsowa AH (2004) Recovery of copper from industrial waste solution by cementation on reciprocating horizontal perforated zinc disc. Desalination 167:127–133
El-Shazly AH, Mubarak AA, Farag HA, Fadl AG (2014) Improving the rate of Cu+2 recovery from industrial wastewater using a vertical array of reciprocating perforated zinc discs. Alex Eng J 54(1):71–76
Nosier SA, Sallam SA (2000) Removal of lead ions from wastewater by cementation on a gas-sparged zinc cylinder. Sep Purif Technol 18(2):93–101
Hsu YJ, Kim MJ, Tran T (1999) Electrochemical study on copper cementation from cyanide liquors using zinc. Electrochim Acta 44(10):1617–1625
Nosier SA (2003) Removal of cadmium ions from industrial wastewater by cementation. Chem Biochem Eng Q 17(3):219–224
Konsowa AH (2010) Intensification of the rate of heavy metal removal from wastewater by cementation in a jet reactor. Desalination 254(1):29–34
Nassef E, El-Taweel YA (2015) Removal of copper from wastewater by cementation from simulated leach liquors. J Chem Eng Process Technol 6(1):1–6
Alemany C, Aurousseau M, Lapicque F, Ozil P (2002) Cementation and corrosion at a RDE: changes in flow and transfer phenomena induced by surface roughness. J Appl Electrochem 32(11):1269–1278
Abdel-Aziz EG, El-Naggar MA, Nosier SA, Mubarak AA, Abdel-Aziz MH, Sedahmed GH (2020) Recovery of copper from industrial waste solutions by cementation using a rotating fixed bed of stacked steel screens. Min Metall Exploration 37:453–458
El-Ashtoukhy E-SZ, Abdel-Aziz MH (2013) Removal of copper from aqueous solutions by cementation in a bubble column reactor fitted with horizontal screens. Int J Miner Process 121:65–69
Grau JM, Bisang JM (2005) Mass transfer studies at rotating cylinder electrodes of expanded metal. J Appl Electrochem 35:285–291
Power GP, Ritchie IM (1975) Modern aspects of electrochemistry. In: Bockris JOM (ed) Conway, BE. Plenum Press, New York
Selley NJ (1977) Experimental approach to electrochemistry. Wiley, London
Kolmachikhina EB, Sviridov AV, Naumov KD (2020) Investigation into the influence of sodium lignosulfonate, anionic surfactants, and their mixtures on the copper cementation rate by zinc. Russ J Non-Ferrous Met 61:488–493
Karavasteva M (1996) The effect of certain surfactants on the cementation of copper by suspended zinc particles. Hydrometallurgy 43:379–385
McCabe WL, Smith JC, Harriott P (2005) Unit operations of chemical engineering. Mc-Graw Hill Inc., New York
Vogel AI (1961) A textbook of quantitative inorganic analysis including elementary instrumental analysis. Wiley, London
Goronja JM, Janošević-Ležaić A, Dimitrijević BM, Malenović A, Stanisavljev D, Pejić N (2016) Determination of critical micelle concentration of cetyltrimethyl-ammonium bromide: different procedures for analysis of experimental data. Hemijska Ind 70:485–492
Rahman A, Brown CW (1983) Effect of pH on the critical micelle concentration of sodium dodecyl sulphate. J Appl Polym Sci 28:1331–1334
Findlay A, Kitchner J (1965) Practical physical chemistry. Longman, London
Lobo VMM (1993) Mutual diffusion coefficient in aqueous electrolyte solutions (technical report). Pure Appl Chem 65(12):2613–2640
Walsh FC (1993) A first course in electrochemical engineering. The electrochemical consultancy. Hants UK
Fahidy Z (1985) Principle of electrochemical reactor analysis. Elsevier, New York
Hung YP, Mohamed N, Darus H (2005) Recovery of copper from strong chloride-based solution. J Appl Sci 5(8):1328–1333
Welty JR, Wicks CE, Rorer G, Wilson RE (2007) Fundamentals of momentum, heat, and mass transfer. Wiley, Hoboken
Lee EC, Lawson F, Han KN (1978) Effect of precipitant surface roughness on cementation kinetics. Hydrometallurgy 3(1):7–21
Ekmekyapar A, Tanaydin M, Demirkiran N (2012) Investigation of copper cementation kinetics by rotating aluminum disc from the leach solutions containing copper ions. Physicochem Problems Min Process 48(2):355–367
Demirkiran N, Kuenkuel A (2011) Recovering of copper with metallic aluminum. Trans Nonferrous Met Soc China 21(12):2778–2782
Tanaka M, Yamashita I, Chisaka F (1990) Flow and heat transfer characteristics of the Stirling engine regenerator in an oscillating flow. JSME Int J Ser 33(2):283–289
Shehata AS, Elshazly AH, Zaatout AA, Sedahmed GH (2011) Mass transfer behaviour of a new liquid-liquid rotating screen disc extractor. Bul Chem Commun 43(3):427–438
Nagamalleswara Rao K, Koteswara Reddy G, Murali Mohan V, RajendraPrasad P, Sujatha V (2011) Studies on ionic mass transfer with coaxially placed twisted tape as turbulent promoter in circular conduits. J Eng Res Stud 2(1):49–61
Wu Y, Hua C, Li W, Li Q, Gao H, Liu H (2009) Intensification of micromixing efficiency in a ceramic membrane reactor with turbulence promoter. J Membr Sci 328(1):219–227
Gaber MM, Fouad YO, Farag HA, Sedahmed GH (2015) Intensification of the rate of diffusion controlled electrolytic oxidation of FeSO4 by turbulence promoters in a stirred tank reactor in relation to wastewater treatment. Chem Eng Res Des 97(May):100–108
Fogler HS (2006) Elements of chemical reaction engineering. Prentice-Hall, Massachusetts
Alberty RA (1960) The foundations of chemical kinetics (Ed: Benson, S. W.). Mc-Graw Hill Inc., New York
Dönmez B, Sevim F, Sarac H (1999) A kinetic study of the cementation of copper from sulphate solutions onto a rotating aluminum disc. Hydrometallurgy 53(2):145–154
Sedahmed GH, Ahmed AM (1989) Effect of surface-active agents on the rate of solid-liquid mass transfer with gas generation at the interface. Chem Eng J 42(1):17–23
Karavasteva M (1996) The effect of certain surfactants on the cementation of copper by suspended zinc particles. Hydrometallurgy 43(1):379–385
Zarraa MA (1996) Effect of surface-active substances on the rate of production of copper powder from copper sulphate solutions by cementation on zinc rods in gas sparged reactors. Hydrometallurgy 41(2–3):231–242
Drweesh MA (2004) Effect of surfactants on the removal of copper from waste water by cementation. Alex Eng J 43(6):917–925
Poulson B (1999) Complexities in predicting erosion-corrosion. Wear 233:497–504
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
The contributing editor for this article was Adam Clayton Powell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Naggar, M.A., Hassan, D.M., Zewail, T.M. et al. Extraction of Copper from Liquid Effluents by Cementation in Agitated Vessels Equipped with Expanded Aluminum Cylindrical Sheets. J. Sustain. Metall. 8, 1318–1329 (2022). https://doi.org/10.1007/s40831-022-00573-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00573-1