Abstract
To evaluate whether lung ultrasound is reliable bedside tool to monitor changes of lung aeration at the early and late stages of ARDS. LUS was performed in ARDS patients that underwent at least two consecutive CT scan at ICU admission and at least 1 week after admission. Twelve fields were evaluated and graded from 0 (normal) to 3 (consolidation). Changes of LUS score in twelve fields (ΔLUStot) and in four ventral (ΔLUSV), intermediate (ΔLUSI) and dorsal (ΔLUSD) zones were calculated at each time points. Three categories were described: Improve (ΔLUS < 0), Equal (ΔLUS = 0) or Worse (ΔLUS > 0). LUS scores were correlated with total changes in lung CT aeration (ΔCTair) and with normally, poorly and not aerated regions (ΔCTnorm, ΔCTpoor and ΔCTnot, respectively). Eleven patients were enrolled. ΔLUStot had significant correlation with ΔCTair (r = − 0.74, p < 0.01). ΔLUSV, ΔLUSI and ΔLUSD showed significant correlations with ΔCTair (r = − 0.66, r = − 0.69, r = − 0.63, respectively; p < 0.05). Compared to Equal, Improve and Worse categories had significantly higher (p < 0.01) and lower (p < 0.05) ΔCTair values, respectively. Compared to Equal, Improve and Worse categories had lower (p < 0.01) and higher (p < 0.01) ΔCTnot values, respectively. LUS score had a good correlation with lung CT in detecting changes of lung aeration.
Similar content being viewed by others
1 Introduction
Chest Computed tomography (CT) is the reference imaging technique for the identification and characterization of lung parenchyma anatomical alterations [1]. In the context of Acute Respiratory Distress Syndrome (ARDS), it has been employed to quantify the loss of tissue aeration [2] due to the non-cardiogenic increase in extravascular lung water [3]. Moreover, CT scan allows to identify ARDS morphology and thus the potential for lung recruitment [2, 4]. In ARDS patients, lung ultrasound (LUS), as compared with CT scan, has shown to be able to assess the loss of lung aeration [5] and to predict ARDS morphology [6]. The role of LUS in following-up on aeration changes over time in this population has not been investigated. Therefore, in the context of a study assessing the accuracy of LUS in identifying ARDS morphology [6], we performed a time course analysis to explore the potential role of LUS in monitoring gain or loss of lung aeration as compared to the gold standard (CT scan). We hypothesized that LUS performed at the bedside would accurately quantify lung aeration changes in ARDS patients over time. The primary endpoint was the correlation between LUS score variations and changes in CT scan percentage of aeration over time. The second end point was to quantify CT scan percentage of aeration changes when LUS improved, worsened or remained the same at early and late stages of ARDS.
2 Materials and methods
2.1 Subjects
Patients admitted to the Turin university hospital’s respiratory intensive care unit (ICU) for ARDS, [7] with an expected requirement for mechanical ventilation of at least 24 h and undergoing chest CT for clinical assessment were enrolled. Patients < 18 years of age, those with a confirm diagnosis of pulmonary fibrosis or with an expected survival of less than 24 h were excluded from the study. Time course analysis of LUS accuracy in detecting aeration change was performed in patients that for clinical reason underwent at least two CT scan with the appropriate timing, defined as follows: Early (ICU admission) and Late (at least 1 week after). The study was approved by the local Ethics Committee (0117126) and informed consent was obtained according to Italian regulation.
2.2 Study protocol
CT scan at ICU entry was required for enrollment and subsequent repeating of the exam was performed based on clinical assessment of treating physicians. Whenever CT was performed, LUS examination was recorded, maintaining the same ventilatory settings and sedation level.
Bedside US evaluation were performed by two observers, trained by board-certified consultants with expertise in point of care ultrasound, as to reach the minimum requirements defined to perform with accuracy a standard bedside lung US [8,9,10].
2.3 Lung ultrasound
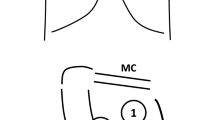
Using the curvilinear transducer (5–3 MHz), with a Mylab Seven ultrasound machine (Esaote S.p.A, Genova, Italy), all the patients were examined in supine position [11]. Twelve LUS fields, six in the left and six in the right hemithorax, were explored basing on the following landmarks [11, 12]: right and left 2nd to 3rd intercostal (IC) spaces across the mid-clavicular (MC) line (fields 1 and 7, respectively); right and left 5th to 6th IC spaces across the MC line (fields 2 and 8); right and left 3rd to 4th IC space across the anterior axillary (AA) line (fields 3 and 9); right and left 6th to 7th IC space across the AA line (fields 4 and 10); right and left 4th to 5th IC space across the posterior axillary (PA) line (fields 5 and 11); right and left 7th to 8th IC space across the PA line and above the diaphragmatic dome (fields 6 and 12). Three regions were identified: ventral (V), intermediate (I) and dorsal (D), corresponding to the zones 1-2-7-8, 3-4-9-10 and 5-6-11-12, respectively. The ultrasound beam was directed transversally along the intercostal space, to insonate the majority of the corresponding lung region as seen on an axial plane [12]. Display depth was set as ≥ 12 cm to correctly interpret US artifacts. The examination lasted the time necessary to give a real time evaluation of the 12 fields. A detailed description of lung regions and anatomical landmarks has been previously reported [6].
According to LUS image characteristics each field was graded as: N (normal aeration): lung sliding/lung pulse with A lines or less than two B lines for intercostal space; B1 (moderate loss of lung aeration): multiple spaced B-lines, more or equal than 3 for each space; B2 (severe loss of lung aeration): multiple coalescent B lines with or without subpleural consolidations; C (consolidation): presence of a tissue pattern with or without air bronchograms [13,14,15,16,17], where N = 0, B1 = 1, B2 = 2, C = 3 [15]. LUS score was directly obtained (real time) and reported by the observer for each field in a dedicated case report form.
The sum of the LUS scores obtained in every 12 fields defined the LUStot variable. The sum of the LUS scores obtained in the four ventral (1-2-7-8), intermediate (3-4-9-10) and dorsal (5-6-11-12) fields defined the variables LUSV, LUSI and LUSD. In order to evaluate lung aeration over time, the relative changes of aeration score in each field (ΔLUS) was calculated for each patient as follows:
where LUS(Early) was the LUS at study entry and LUS(Late) was the LUS obtained at least at one week after. In the same way, the relative changes of LUStot, LUSV, LUSI and LUSD scores were calculated for each patient as follows:
Basing on lung aeration changes identified by ΔLUS, three categories has been described:
-
(1)
“Improve” category: lung aeration improved if ΔLUS was < 0
-
(2)
“Equal” category: lung aeration did not change ΔLUS was = 0
-
(3)
“Worse” category: lung aeration worsened if ΔLUS was > 0
2.4 Lung computed tomography
CT scan was performed at study entry and repeated based on clinical evaluation. Lung aeration at CT scan was assessed blindly with quantitative analysis using a dedicated software (Maluna, University of Mannheim, Germany) [18, 19] in twelve regions of interests (ROI), corresponding to the left and right lung in six CT slices, identified as the areas corresponding to the LUS fields based on pre-defined anatomical landmarks. Sternal manubrium apex/ clavicle: zones 1 (right) and 7 (left); pulmonary trunk, 2 cm below the tracheal carina: zones 2 (right) and 8 (left); lower third of the sternal manubrium, 2 cm beneath the zones 1 and 7, in correspondence with the aortic arch and the scapula: zones 3 (right) and 9 (left); the middle of the sternal body, at the heart base: zones 4 (right) and 10 (left); the lower third of the sternal manubrium, in correspondence with the tracheal carina: zones 5 (right) and 11 (left) and 2 cm above the diaphragm were zones 6 (right) and 12 (left) [6]. The analysis of the selected region of interest (ROIs) with the Maluna software is based on the ray attenuation of each pixel expressed in Hounsfield units (HU) and defined as: hyperinflated (− 900 and − 1000 HU); normally aerated (− 900 and − 500 HU); poorly aerated (− 500 and − 100 HU); and non-aerated (− 100 and 100 HU) [19]. Percentage of aeration (Pair) was derived from Hounsfield units for each ROI, as follows:
where Voltot represents the total volume (in mL) and Volair the volume occupied by air (in mL) in the considered ROI. Percentage of normally (Pnorm), poorly (Ppoor) and not (Pnot) aerated lung where derived as follows:
where Volnorm, Volpoor and Volnot represent the amount in mL of normally, poorly and not aerated lung over the total ROI volume. As previously described for LUS evaluation, changes over time of lung CT densities (ΔCTair) were calculated as follows:
where (Late) and (Early) were the same time points as previously described.
Lung aeration improved if ΔCTair was greater than 0%, whereas lung aeration did not change or worsened if ΔCTair was equal to or less than 0%. Changes over time of Pnorm, Ppoor and Pnot in each ROI were calculated as follows:
where (Late) and (Early) were the same time points as previously described. Changes over time of Pair, Pnorm, Ppoor and Pnot for the entire lung were calculated as the median of ΔCTair, ΔCTnorm, ΔCTpoor and ΔCTnot obtained in all the twelve ROI, respectively. Similarly, changes over time of Pair, Pnorm, Ppoor and Pnot for the ventral, intermediate and dorsal zones were calculated as the median of ΔCTair, ΔCTnorm, ΔCTpoor and ΔCTnot obtained in all the four ventral intermediate and dorsal ROI, respectively.
2.5 Statistics
Data were tested for normal distribution by Shapiro–Wilk test and were expressed as mean and standard deviation (SD) or median with interquartile range 25–75 (IQR), as appropriate.
The relationship between median ΔCTair, ΔCTnorm, ΔCTpoor, ΔCTnot and ΔLUS was evaluated overall and in ventral, intermediate and dorsal regions by Pearson’s correlation coefficient with a null hypothesis that the correlation coefficient was equal to zero; p < 0.05 was considered statistically significant. Comparison between the early and late phase of ARDS was performed with Wilkcoxon test for paired samples. Kruskal–Wallis one-way analysis of variance with post-hoc Dunn’s test for multiple comparison was performed to compare ΔCTair, ΔCTnorm, ΔCTpoor, ΔCTnot between categorical changes of ΔLUS. To obtain an 80% power to detect a negative correlation between ΔLUStot and ΔCTair (primary endpoint) of at least − 0.75, with an alpha error of 0.05 (one sided), a sample size of 10 participants was required. Statistical analyses were performed using Stata 13.1/SE (Stata Corporation, Texas, USA).
3 Results
3.1 Study population
Eleven patients with ARDS were enrolled in the study. Twenty-two lung CT scans were performed corresponding to twenty-two LUS examinations. A total of 132 LUS fields for each time point (Early VS Late) were evaluated, corresponding to an equal number of CT ROIs. Each ventral, intermediate and dorsal region encompassed a total of 44 fields/ROIs. Median age was 52 years (44–58), 7 (64%) were male, Severity Acute Physiologic Score (SAPSII) and Sequential Organ Failure Assessment (SOFA) score at ICU admission were 42 (34–51) and 10 (8–11), respectively. ARDS aetiology was bacterial pneumonia in 6 (55%), viral pneumonia in 4 (36%) and abdominal sepsis in 1 (9%) patients. Five (45%) and six (55%) patients over eleven died after 28 and 60 days from ICU admission, respectively. ICU length of stay was 29 (19–45) days. Clinical data of the included patients at Early and Late timepoints are reported in Table 1S.
3.2 Monitoring of lung aeration over time
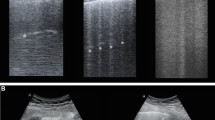
Representative lung CT and corresponding LUS images in ventral, intermediate and dorsal lung regions at early and late stages of ARDS in two opposite cases of aeration improvement or worsening are shown in Fig. 1 (panel A and B). Absolute total and regional LUS score and Pair, Pnorm, Ppoor and Pnot values at Early and Late time points are described in Table 1S.
Correlations between median ΔCTair, ΔCTnorm, ΔCTpoor, ΔCTnot and ΔLUS (N = 11) are described in Table 1. Increments in ΔLUS were significantly inversely related to median ΔCTair decrease both globally (ΔLUStot r = − 0.74, p < 0.01) and in ventral (ΔLUSV r = − 0.66, p < 0.05), intermediate (ΔLUSI r = − 0.69, p < 0.05) and dorsal (ΔLUSD r = − 0.63, p < 0.05) regions. Similarly, ΔLUS was positively correlated to median ΔCTnot both globally (ΔLUStot r = 0.79, p < 0.01) and in ventral (ΔLUSV r = 0.76, p < 0.01), intermediate (ΔLUSI r = 0.74, p < 0.01) and dorsal (ΔLUSD r = 0.62, p < 0.05) regions. Median ΔCTpoor showed poor or no correlations with corresponding LUS variations. Median ΔCTnorm was significantly associated to ΔLUStot and ΔLUSI (r = − 0.67, p < 0.05 and r = − 0.66, p < 0.05, respectively).
Lung CT density variations, in Early and Late time points over categorical changes in LUS score are shown in Table 2. In Fig. 2 the changes in median ΔCTair, ΔCTnorm, ΔCTpoor and ΔCTnot are shown based on the corresponding changes in categorical ΔLUS score for each field. Percentage of aeration at CT scan significantly reduced in the fields where lung aeration worsened according to LUS score [19% (− 28–4)], compared to the fields in which LUS score remained the same [− 2% (− 15–4), p < 0.0385] or improved [6% (− 5–30), p = 0.0000] and in the fields in which LUS score remained the same [− 2% (− 15–4)] compared to the ones in which LUS score improved [6% (− 5–30), p = 0.0029–panel A]. Similarly, there was a significant increase in terms of amount of ΔCTnot in the fields where LUS score worsened [35% (− 1–42)] compared to the fields in which LUS score remained the same [− 1% (− 6–13), p = 0.0049] or improved [− 8% (− 32–2), p = 0.0000] and in the fields in which LUS score improved [− 8% (− 32–2)] compared to the ones where LUS score remained the same [− 1% (− 6–13), p = 0.0043–panel D]. Increments in ΔCTnorm were significant only in the fields where LUS score improved (10% (− 4–43)) compared to the ones in which LUS score remained the same [− 2% (− 22–9), p = 0.0030] or worsened [− 24% (− 30–19), p = 0.0000–panel B]. Conversely, a reduction in ΔCTpoor was statistically significant only in the fields where LUS score improved [1% (− 8–6), p = 0.0020] or remained the same [2% (− 5–7), p = 0.0002] compared to the fields where LUS score worsened [− 8% (− 14–4)–panel C].
The Spearman correlation coefficient field by field between ΔLUS and ΔCTair was – 0.42 (p < 0.0001, n = 128). Spearman correlation coefficient between ΔLUS and ΔCTnorm and ΔCTnot were – 0.40 (p < 0.05) and 0.48 (p < 0.0001), respectively.
4 Discussion
The main findings of this study is that LUS had a good correlation with lung computed tomography analysis in detecting changes of lung aeration when performed at early and late stages of ARDS. Improving or worsening of LUS was associated with higher percentage of normally and not aerated regions of lung computed tomography, respectively. Recently, Chiumello et al. showed that LUS is a valuable bedside tool to evaluate lung tissue aeration, although it should probably not be used to assess alveolar recruitment [5]. In ARDS patients supported with extra-corporeal membrane oxygenation (ECMO), LUS has shown to be a valid tool for daily monitoring of aeration [20]. In addition, lung ultrasound has been demonstrated to be a useful method to evaluate changes in extravascular lung water in patients with ARDS [21] and acute kidney injury requiring renal replacement therapy and pulmonary congestion of patients with chronic heart failure [22]. The number of B lines was also associated with net negative fluid balance after dialysis [22] and with hospital length of stay and mortality in a cohort of ambulatory heart failure patients [23]. Recently, LUS performed directly on the lung surface has been used to monitor lung aeration changes over time and as a prognostic tool in lung donor in the context of the normothermic ex-vivo lung perfusion (EVLP) [24, 25]. Our study highlights for the first time that LUS, compared with the gold standard CT, was able to detect even small changes in percentage of aeration (ΔCTair) over time, suggesting that LUS may represent an accurate bedside and radiation-free tool to monitor and quantify the degree of ARDS resolution or worsening. In addition, our study shows that LUS performed better in recognizing changes of not aerated (ΔCTnot) tissue. This finding could be explained with the fact that LUS accuracy relies basically on the presence/absence of B lines or consolidation, which are predominant in not aerated tissue. Resolution of consolidation pattern (e.g. from consolidation to few B lines or A lines) is easily recognized by LUS. Regarding LUS performance in recognizing changes in normally aerated tissue, a possible explanation consists in the fact that A lines artifact is the hallmark for normally aerated tissue, and the emergence of B lines is easily identified without much false positive. Conversely, it is impossible for LUS to distinguish between overinflated tissue and normal aeration. At the same time, comparing a categoric ordinal variable as LUS score with a continuous one, is probably the rationale to explain the limitation of LUS in discriminating the faceted aspects of a poorly aerated slice.
Following up ARDS radiological changes could be challenging in the ICU because of the low accuracy of bedside anterior–posterior chest X-ray in defining ARDS morphology and the distribution of lung aeration loss [26, 27]. CT scan remains the gold standard for monitoring lung aeration changes, but it could be harmful in daily practice because of the risks related to patient mobilization and ionized radiations. Patients with severe ARDS can be burdened during the transfer from the ICU to the CT scan facility by several adverse events which might compromise oxygenation, such as accidental disconnection from the ventilator with lung de-recruitment. In addition, patients with ECMO support can be exposed to severe complications such as cannulas misplacement and pump malfunction that negatively contribute to patient’s outcome. Lung ultrasound might represent therefore an accurate and cost effective bedside tool for monitoring lung aeration changes over time. In addition, the quantitative lung aeration assessment with dedicated software that we used in this study (e.g. Maluna® or analogues) could not be widely available in every ICU, thus limiting CT scan efficacy in evaluating lung parenchyma aeration in daily practice.
This study has some limitations. First, the different spatial resolution of CT and LUS may influence lung aeration assessment [6]. In fact, different from LUS, CT scan analysis encompasses the whole thoracic area from along the chest wall to mediastinal organs. Second, this study was a pilot study with a small sample size; further external validation is warranted. Third, the whole analyses were performed on ARDS patients before the COVID-19 pandemic. Therefore, we can’t extend our findings to patients with COVID-19 associated ARDS. Fourth, B-lines were quantified by real time counting, without using have automatic B line quantification. This might have affected overall accuracy [28].
5 Conclusions
In conclusion, in our cohort of ARDS patients, LUS was a reliable bedside tool to monitor changes of lung aeration overtime when compared to the gold standard lung computed tomography. This technique might be employed in patients with ARDS to reliably assess the healing or not of the lung parenchyma. Future research is required to confirm the utility of this technique in larger studies.
Change history
16 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10877-024-01137-2
References
Chiumello D, Papa GFS, Artigas A, Bouhemad B, Grgic A, Heunks L, et al. ERS statement on chest imaging in acute respiratory failure. European Respiratory Journal [Internet]. European Respiratory Society; 2019. 54. http://erj.ersjournals.com/content/54/3/1900435. Accessed 2 Jul 2021
Constantin J-M, Grasso S, Chanques G, Aufort S, Futier E, Sebbane M, et al. Lung morphology predicts response to recruitment maneuver in patients with acute respiratory distress syndrome. Crit Care Med. 2010;38:1108–17.
Berkowitz DM, Danai PA, Eaton S, Moss M, Martin GS. Accurate characterization of extravascular lung water in acute respiratory distress syndrome. Crit Care Med. 2008;36:1803–9.
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–86.
Chiumello D, Mongodi S, Algieri I, Vergani GL, Orlando A, Via G, et al. Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients. Crit Care Med. 2018. https://doi.org/10.1097/CCM.0000000000003340.
Costamagna A, Pivetta E, Goffi A, Steinberg I, Arina P, Mazzeo AT, et al. Clinical performance of lung ultrasound in predicting ARDS morphology. Ann Intensive Care. 2021;11:51.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33.
Arbelot C, Dexheimer Neto FL, Gao Y, Brisson H, Chunyao W, Lv J, et al. Lung Ultrasound in emergency and critically Ill patients: number of supervised exams to reach basic competence. Anesthesiology. 2020;132:899–907.
Bronshteyn YS, Anderson TA, Badakhsh O, Boublik J, Brady MBW, Charnin JE, et al. Diagnostic point-of-care ultrasound: recommendations from an expert panel. J Cardiothorac Vasc Anesth. 2022;36:22–9.
Russell FM, Ferre R, Ehrman RR, Noble V, Gargani L, Collins SP, et al. What are the minimum requirements to establish proficiency in lung ultrasound training for quantifying B-lines? ESC Heart Fail. 2020;7:2941–7.
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–91.
Mongodi S, Bouhemad B, Orlando A, Stella A, Tavazzi G, Via G, et al. Modified lung ultrasound score for assessing and monitoring pulmonary aeration. Ultraschall Med. 2017. https://doi.org/10.1055/s-0042-120260.
Bouhemad B, Liu Z-H, Arbelot C, Zhang M, Ferarri F, Le-Guen M, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38:84–92.
Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby J-J. Bedside ultrasound assessment of positive end-expiratory pressure–induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–7.
Soummer A, Perbet S, Brisson H, Arbelot C, Constantin J-M, Lu Q, et al. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40:2064–72.
Lichtenstein DA, Mezière GA, Lagoueyte J-F, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest J. 2009;136:1014–20.
Tierney DM, Huelster JS, Overgaard JD, Plunkett MB, Boland LL, St Hill CA, et al. Comparative performance of pulmonary ultrasound, chest radiograph, and CT among patients with acute respiratory failure. Crit Care Med. 2020;48:151–7.
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–6.
Grasso S, Terragni P, Mascia L, Fanelli V, Quintel M, Herrmann P, et al. Airway pressure-time curve profile (stress index) detects tidal recruitment/hyperinflation in experimental acute lung injury. Crit Care Med. 2004;32:1018–27.
Mongodi S, Pozzi M, Orlando A, Bouhemad B, Stella A, Tavazzi G, et al. Lung ultrasound for daily monitoring of ARDS patients on extracorporeal membrane oxygenation: preliminary experience. Intensive Care Med. 2018;44:123–4.
Zhao Z, Jiang L, Xi X, Jiang Q, Zhu B, Wang M, et al. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm Med. 2015;15:98.
Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJR, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time Course for Resolut Chest. 2009;135:1433–9.
Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J. 2016;37:1244–51.
Ayyat KS, Okamoto T, Niikawa H, Sakanoue I, Dugar S, Latifi SQ, et al. A CLUE for better assessment of donor lungs: novel technique in clinical ex vivo lung perfusion. J Heart Lung Transplant. 2020. https://doi.org/10.1016/j.healun.2020.01.981.
Costamagna A, Steinberg I, Simonato E, Massaro C, Filippini C, Rinaldi M, et al. Clinical performance of lung ultrasound in predicting graft outcome during ex vivo lung perfusion. Minerva Anestesiol. 2021. https://doi.org/10.23736/S0375-9393.21.15527-0.
Rouby J-J, Puybasset L, Nieszkowska A, Lu Q. Acute respiratory distress syndrome: lessons from computed tomography of the whole lung. Crit Care Med. 2003;31:S285.
Rouby J-J, Puybasset L, Cluzel P, Richecoeur J, Lu Q, Grenier P, et al. Regional distribution of gas and tissue in acute respiratory distress syndrome. II. Physiological correlations and definition of an ARDS Severity Score. Intensive Care Med. 2000;26:1046–56.
Pičuljan A, Šustić M, Brumini G, Kuharić J, Šustić A. Reliability of B-line quantification by different-level observers and a software algorithm using point-of-care lung ultrasound. J Clin Monit Comput. 2020;34:1259–64.
Acknowledgements
The authors thank prof Michel Quintel who made available MALUNA software for lung CT images analysis.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AC: Study design, data interpretation and analysis and manuscript writing and revision. PE: Study design, data analysis and manuscript revision. SI: Data collection and analysis, manuscript revision. AP: Data collection and analysis, manuscript revision. VS: Study design, data collection and manuscript revision. BL: Data interpretation and manuscript revision. FV (corresponding author): Study design, data analysis and interpretation, manuscript writing and revision. All the authors approved the final version of the manuscript to be published and agreed to be accountable for all aspects of the work. Prof. Vito Fanelli is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to ethics, to published article.
Corresponding author
Ethics declarations
Conflict of interest
The authors report the absence of conflicts of interest related to the submitted work.
Ethical approval
This observational study was approved by the local Ethics Committee (0117126).
Informed consent
Informed consent was obtained according to Italian regulation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: to correct, the given and family names of all the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Costamagna, A., Steinberg, I., Pivetta, E. et al. Clinical performance of lung ultrasound in predicting time-dependent changes in lung aeration in ARDS patients. J Clin Monit Comput 37, 473–480 (2023). https://doi.org/10.1007/s10877-022-00902-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00902-5