Abstract
The study aimed at assessing the spring water within Igbo-Etiti, Nigeria, for drinking and irrigation applications using physicochemical parameters, water quality index, mineral and trace elements, pollution indices, and risk assessment. Standard methods were used to determine the physicochemical properties of the spring water in rainy and dry seasons. Trace metals such as Pb, Cd, Zn and Cu were determined with atomic absorption spectrophotometer. The results showed that most of the physicochemical properties studied were within the guideline values set by Nigeria Standard for Drinking Water Quality (NSDWQ), WHO and US EPA for drinking water purposes. However, pH of all the spring water (4.27–4.73; and 4.95–5.73) for dry and rainy seasons, respectively, were slightly acidic. Lead (Pb) (0.01–1.08 mg/L) and cadmium (Cd) (0.01–0.15 mg/L) concentrations were above the guideline values in both seasons. This could be attributed to the lithology of the study area which is Nsukka FORMATION. Leaching of lead and sulphides from the embedded coal deposits could be responsible for the increased lead levels and made the water acidic. The mineral and trace elements examined were within WHO and NSDWQ limits for drinking water except Cd and Pb. The permeability index and other irrigation indices showed that all the spring water was suitable for irrigation purposes. The pollution index suggested that the pollution level of all the samples in the dry season was insignificant (PIS < 1). Two-way ANOVA showed significant differences in most of the parameters studied in the dry and rainy seasons. Pearson correlation analysis and cluster analysis showed strong significant positive and negative correlations in some of the parameters studied in both seasons. The water quality index showed that none of the spring water had excellent water status. However, one spring (Iyi Ase) had poor water status in dry season and is considered unsafe for drinking. Iyi Ase was also considered not suitable for irrigation application as predicted by most of the pollution indices, while others were generally considered suitable for irrigation application. Probable cancer and non-cancer risk assessment revealed a probable risk associated with the consumption of the spring in Igbo-Ettiti area, Nigeria.
Similar content being viewed by others
Introduction
Water resources are significant due to the cultural, social, and economic benefits which they provide for the society and being a crucial support for the biodiversity of the ecosystems (Rosca et al. 2020; Dippong et al. 2019). The water quality has a great impact on health and, certainly, on life (Rosca et al. 2020). The overuse of water, climate change, and pollution are threatening factors for the natural and limited water resources such as springs (Rosca et al. 2020).
Goldscheider et al. (2020) report that 15.2% of the global land surface is covered by karstic terrains, and freshwater springs are known to supply partially or entirely 25–50% of drinking water to the world’s population (Hartmann et al. 2014). United Nations Sustainable Development Goal (SDG) 6 is to ensure access to safe drinking water and sanitation for all by 2030 (United Nations 2018), and proper water and sanitation are critical to achieving SDGs of good health and gender equality.
Springs are widely distributed across landscapes and sustain and support millions of people across the globe including in Nigeria (Bhat et al. 2020, 2022). Spring is a point at which water flows from an aquifer to the earth’s surface. It is also a point in the hydrological cycle when underground water enters the land surface and afterward flows into a stream (Water science school, 2018; Chukwu, 1999). A spring may be the result of karst topography where surface water has infiltrated the earth’s surface (recharge area), becoming part of the area groundwater. The quality of the water at the surfacing point is usually good as the water has penetrated through thick strata of soil. The water picks dissolved minerals (such as magnesium, calcium and iron, etc.), during the percolation process and is purified of biological organisms, (disease-producing pathogens) example bilharzias FAO (2002).
The quality of spring water can be altered by geochemical reactions, agricultural activities (Ehiemere et al. 2022), saline water intrusion, improper waste disposal (Alum et al. 2021), urbanization, and others that are classed as natural or anthropogenic (Akhtar et al. 2014). Spring water quality monitoring on regular basis, most importantly in areas with a high risk of pollution, is paramount for the protection of the rural dweller’s health especially in developing countries like Nigeria. Jones et al. (2014) noted that the strategies for the assessment of spring water or groundwater quality generally are purported at establishing the present status and appraisal of environmental parameter trends. These enhance the determination of the level of danger the polluted spring water could pose to the rural dwellers in particular and the environment in general. The chemical composition of groundwater is influenced and altered by the fast development of society (urbanism, industrialization, mining activities, radioactive activities, domestic and agricultural sources) and also by natural processes (volcanism, weathering of rocks, evaporation processes) implicitly affecting the human health and the natural environment (Dippong et al. 2020; Rosca et al. 2020; Dippong et al. 2018).
Besides, the danger associated with water is heterogeneous and differs from one locality or region to another, and so, assessment and investigation need to be local or regional as well (Biswas & Tortajada, 2010). In recent time, countries in sub-Saharan Africa have made a substantive improvement in the provision of potable water to their populace to which about 43% has access to. However, this is through private boreholes that are usually not monitored or assessed regularly (WHO/UNICEF, 2015). Nigeria has a population of over 205 million people (Worldometer, 2020) and is still among the countries in sub-Saharan Africa battling with the provision of potable water to its teaming citizens. This has led its citizens to seek for alternatives (spring and other groundwaters) given the near-zero pipe-born water scheme in the country (Owamah et al. 2013; Dahunsi et al. 2014).
Igbo-Etiti is one of the local government areas (LGA) in Enugu state and as is the case in Enugu state generally, the major source of drinking water is the springs and sometimes boreholes for those that can afford to sink one or connect from the state government-owned water system. In recent times, the cultivation of vegetables and other crops around the springs in the area has been on the increase and the quality of the agricultural output has been questionable as a result of the activities that go on in and around the springs in Igbo-Etiti which are assumed to be polluted. Besides, the pollution of these main sources of water for the rural populace poses a serious threat to the health of the rural dwellers in Igbo-Etiti as they are used without any kind of treatment. This research aims to assess the quality of spring water resources in Igbo-Etiti, Nigeria for drinking and irrigation application. Due to the increasing applications of spring water as an alternative to pipe-borne water schemes, for drinking, irrigation, and domestic uses in Igbo-Etiti, Enugu state, Nigeria, there is a need for frequent appraisal of the spring water in the area, hence, the need for this study.
Materials and methods
Study area
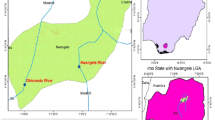
Enugu state is one of the 36 states of Nigeria of which Igbo Etitiis a local government among the 17 local government areas in Enugu state (Fig. 1). It is located in the southern part of Enugu North, southeast of Nigeria, and it is bounded between latitude 6º36´N to 6º47´N and longitude 7º15´E and 7º32´E (Ofomata 1978). Its notable towns are Ekwegbe, Ukehe, Ogbede and Aku. The sampling points are quite accessible due to good networks of minor and major roads. The road network links Ninth-mile corner and subsequently Onitsha which are popular towns in the state and neighbouring state, respectively. The area is known by its undulating hills and enormous low-lying plains with height fluctuating from about 50 to more than 400 m above sea level (Ifediegwu et al. 2019). There are many rivers (Adada, Ajali, Obinna and Karawa) traversing the study area. The Adada River is a significant tributary of River Niger, while Obinna is a significant tributary of Anambra River. The inhabitants of these LGAs depend on streams, lakes, springs, rivers and shallow hand-dug wells, although there are few private boreholes (Alum and Okoye 2020). The study area has two distinct climatic seasons; dry and rainy seasons. The dry season begins around October, while the rainy season starts around April. These seasons are caused by the north–south fluctuations of discontinuity between the dry continental (Sahara) air and the humid Atlantic air (Ifediegwu et al. 2019). The Harmattan period is characterized by cold, dry and windy weather, while the rainy season is characterized by heavy, intense rainfall (Iloeje1995). The major agricultural products in the area are rice, yam, banana, maize, cassava, okra and pineapple.
Possible sources of contamination to these spring water
The topographic condition, as well as the anthropogenic activities, 30 m around the springs was taken into consideration at the time of sampling since they have a high tendency of contributing to the levels and types of contaminants of the springs. Indiscriminate dumping of refuse, defecation around the water sources especially on the uphill side of the springs and the agricultural activities can be linked to spring water contamination. The members of these communities around these spring water sources engage in these activities such as the use of animal waste as fertilizer for agricultural activities than inorganic fertilizers and this may also cause spring water contamination. During raining season, all these pollutants will likely be washed down along with free-ranging cattle droppings from the upstream by runoff to contaminate the springs. All these pose the threat of contamination of these spring waters.
Geo-hydrology of the study area
The underlying geologic formations are mainly three (Ajali, Nsukka and Imo FORMATIONS) which are part of the Anambra and Niger Delta sedimentary basins (Ifediegwu et al. 2019). The main underlying geology is predominantly sandstone (Ajali and Nsukka). This is a result of the lithography of the area which is chiefly Nsukka FORMATION. The lithology of Nsukka FORMATION has mainly interbedded shales, siltstone shale, sandy shale and thin coal seams which have become lateralized in many places where they characteristically form resistant capping on mesas and buttes (Akande et al. 2007). The Ajali Sandstone FORMATION is considered a good aquifer and there is existing groundwater exploitation (although not necessarily in the study town). The sandstone is thick and has high values of hydraulic conductivity, and specific yields have been reported (Obasi et al. 2013). The aquifer properties imply that it could be possible to abstract good yields without excessive drawdown of water levels. There are relatively few surface water features because of the permeable surface geology: spring-fed streams are often ephemeral (Lesley and Phil 2009).
Quality assurance procedure
The polyethylene sampling containers and standard flasks were soaked in water and detergent for at least 6 h and rinsed with deionized water and with a mixture consisting of 520 mL de-ionized water, 200 mL concentrated HCl and 80 mL H2O2 Yousif AM (2019). They were then rinsed with de-ionized water and then turned upside down on a clean surface to dry without contamination.
Sample collection and preservation
Five spring water locations were identified in the study area (Aku and Ochima), and water samples were collected seasonally (dry and rainy seasons). A total of thirty (30) water samples comprising of three samples from each spring for each season were collected from the five different springs in January to early February and July to August 2019, representing the dry and rainy seasons, respectively. Each sample was collected with a clean two liter screw-capped polyethylene container which was thoroughly washed with detergent, rinsed with analytical grade 1:1 HCl/deionized water and finally rinsed with deionized water. At the point of water collection, the polyethylene containers were rinsed three times with the water samples. The polyethylene containers were positioned at the point source of the spring water to minimize the contamination of water samples by surface films. The polyethylene was filled leaving no air space and immediately covered. Temperature and pH of the samples were determined on the spot and the samples were transported to the laboratory in a cool box at a temperature of 4 °C to maintain the concentrations of the analytes. The stored samples were analysed within 4 days of collection.
Water quality index (WQI)
The water quality index is used to assess the combined effect of individual parameters on the prevailing quality of water. In all, twelve (12) essential parameters were selected for the water quality index evaluation. Evaluation of the WQI of the spring water samples was done using the weighted arithmetic index method by Chatterji and Raziuddin (2002), which is basically on the studied values of the physical parameters. WHO guidelines were used to assess the quality of the different water samples. The WQI was calculated once for each season (dry and raining, respectively). The water quality status, according to Chatterji and Raziuddin, (2002), was classified as WQI of 0–25 (excellent), 26–50 (Drinking, Irrigation and Industrial), 51–75 (Irrigation and Industrial), 76–100 (irrigation), and more than 100 (Proper treatment required before use).
Determination of physicohemical parameters
Turbidity, sulphate, total dissolved solids (TDS), total suspended solids (TSS), nitrates, total alkalinity, phosphate, total hardness, pH (Jenway pH meter, model 3510), conductivity (WTW Conductivity Meter, Model LF.90) and odour were analysed according to APHA, (2005) standard methods.
Sodium and potassium concentration
Flame photometer (Sherwood 410 model) was used to determine sodium (Na) and potassium (K). Blanks were aspirated and blanks control were adjusted to show 0.0. The highest standard was aspirated, and the coarse and fine control were adjusted to show 200 for 20.0 mg/L, followed by the quality control standard (10.0 mg/L) to check recovery. Thereafter, other standards in ascending order were aspirated and then followed by samples. However, their concentrations were determined against their intensities extrapolated from the graph. 10 mL of water sample was measured into a 10 mL sample cell. The sample was nebulized into the off flame, and the emission intensity was recorded (Table 1).
Pollution index of the spring water
According to Rao (2012), the pollution index of spring water (PIS) is a scale of numbers used to express the extent to which drinking water is polluted. It shows, in a non-ambiguous way, the extent to which individual water chemical variables affect the overall quality of water. Although the PISmodel was initially applied for groundwater quality assessment (Rao 2012; Egbueri and Unigwe 2019; Egbueri et al. 2020), it was applied in the current study for spring water. This decision was based on the fact that the same parameters were analyzed for all the samples. Moreover, all the samples were analyzed under the same set of conditions. Five steps were involved in determining the PIS of a water sample. In the first step, a relative weight (Rw) ranging from 1 to 5 was allotted to each water quality variable (Table 2). The weight assignment was based on the extent to which such a variable impacts human health. In the second step, the weight parameter (Wp) for each water quality measure is calculated using Eq. 4 (through Eqs. 1, 2 and 3) to get the relative share of each water quality measure on the overall water quality. It is the ratio of Rw of every water quality measured to the sum of all relative weights (Eq. 4).
The third step involved working out the status of concentration (Sc) of water quality measure of each water sample to its drinking water quality standard. The Sc was computed by dividing the concentration (C) of each water quality measure of every water sample by its respective drinking water quality standard (Ds) (Eq. 2.
The fourth step involved deriving the overall water quality by multiplying Wp of each water quality measure with its corresponding Sc (Eq. 3.
The final step was the computation of PIS by adding all values of Ow contributed by all water quality measures of every water sample as described in Eq. 4.
Based on the PIS scores, water samples can be classified as having insignificant pollution (PIS < 1), low pollution (PIS of 1.0 and 1.5), moderate pollution (PIS of 1.5–2.0), high pollution (PIS of 2.0–2.5) and very high pollution (PIS > 2.5) (Rao, 2012).
Trace metals analysis
Atomic Adsorption Spectrometer (Model AA-7000 Shimadzu) equipped with air-acetylene flame was used to determine the concentrations of the trace metals. All the glasswares used were soaked in 3 M HNO3 overnight and washed with de-ionized water to reduce the chances of interferences. All the reagents used were of analytical grade and 30 cm3 of aqua regia (HNO3 and HCl in 1:3 ratio) was used for the sample digestion.
Water quality assessment for irrigation purposes
In the evaluation for the usability of the spring water for irrigation, the following water quality indices were used:
-
1.
Permeability index (PI) according to Doneen (1964) was applied to classify the spring water for irrigation application. The PI equation is
$$\mathrm{PI }=({(\mathrm{Na}}^{+ }+ \surd {\mathrm{HCO}}_{3}^{-})/{\mathrm{Na}}^{+}+ {\mathrm{Mg}}^{2+ }+ {\mathrm{Ca}}^{2+}) \times 100$$(5) -
2.
Doneen (1964) classification pattern using soluble sodium percent (SSP) was also used to evaluate the spring water and the equation is written as
$$\mathrm{SSP}=\frac{{\mathrm{Na}}^{+ }+ {\mathrm{K}}^{+}}{{\mathrm{Na}}^{+}+ {\mathrm{Ca}}^{2+}+ {\mathrm{Mg}}^{2+}{+\mathrm{ K}}^{+ }}\times 100$$(6)An SSP of about 60% could result in sodium accumulations that will lead to a breakdown in the soil’s physical properties.
-
3.
The classification according to Kelly’s ratio was used (Kelly 1940) as in Eq. 7.
$$\mathrm{KR}= {\mathrm{Na}}^{+ }/({\mathrm{Ca}}^{2+ }+ {\mathrm{Mg}}^{2+})$$(7) -
4.
The irrigation water classification using magnesium adsorption ratio (MAR) as proposed by Raghunath (1987) was also used as in Eq. 8:
$$\mathrm{MAR}= {(\mathrm{Mg}}^{2+}/({\mathrm{Ca}}^{2+ }+ {\mathrm{Mg}}^{2+})) \times 100$$(8) -
5.
Residual sodium carbonate (RSC) or Residual alkalinity (RA) as suggested by Richards (1954) and Eaton (1950):
$$\mathrm{RSC}={(\mathrm{HCO}}_{3}^{-}+ {\mathrm{CO}}_{3})-({\mathrm{Ca}}^{2+ }+ {\mathrm{Mg}}^{2+})$$(9) -
6.
Chloro-alkaline indices (CAI) are defined as
$$\mathrm{CAI}=\frac{{\mathrm{Cl}}^{-}-\left({\mathrm{Na}}^{+}+ {\mathrm{K}}^{+}\right)}{{\mathrm{Cl}}^{-}}$$(10) -
7.
Richards (1954) also proposed sodium adsorption ratio (SAR) to classify water irrigation as noted in Eq. 11:
$$\mathrm{SAR}=\frac{\mathrm{Na}}{\sqrt{(\mathrm{Ca}+\mathrm{Mg})/2}}$$(11)
An indication of the level to which irrigation water undergoes cation exchange reaction in the soil is given by SAR.
Percent sodium
The permeability of soil is reduced when Na reacts with it (Todd, 1980). Sodium content is usually expressed in terms of percent sodium (sodium percentage), defined by
%Na values classify water as excellent (< 20), good (21–40), permissible (41–60), doubtful (61–80) and unsuitable (> 81) (Wilcox 1955).
Noncarcinogenic and carcinogenic health risk assessment
The non-carcinogenic health risks associated with the consumption of water contaminated by trace elements have been reported by many authors (Egwuonwu et al. 2021; Zhang et al. 2018; Ukah et al. 2019; Ezugwu et al. 2019; Egbueri, 2020a).
Chronic daily intake (CDI)
The chronic daily intake (CDI in mg/kg/day) risks associated with ingesting a trace metal were calculated for both adult and children population using Eq. 13 (US-EPA 1999).
where Cw = concentration of trace element in water (mg/L); IRW = ingestion rate of water (2 L for adults and 1 L for children); EF = the exposure frequency (365 days per year); ED = exposure duration (children = 6 years and adult = 70 years); BW is the body weight (15 kg and 70 kg for children and adult, respectively); AT = average exposure time (70 years * 365 days and 6 years × 365 days for adult and children, respectively) (Barzegar et al. 2018; Ihedioha et al. 2019, 2016; Duggal et al. 2017; Mgbenu and Egbueri, 2019; Egbueri, 2020a).
Hazard quotients (HQ)
The non-carcinogenic risk for each trace element was calculated as hazard quotient using Eq. 14:
where RfD = oral reference dose of a particular trace element (mg/kg/day), CDI = chronic daily intake. The RfD for Cu = 0.04, Cd = 0.001 and Pb = 0.004, mg/kg/day (U.S. EPA 2011, 1999; Egwuonwu et al. 2021; Oluwasola et al. 2021; Nwoke et al. 2021; Odum et al. 2021).
Hazard index (HI)
The summation of the HQ gives the HI of each trace element’s non-carcinogenic risk
If HI is greater than 1, it indicates that ingestion of trace elements could have a non-carcinogenic detrimental effect. Therefore, on the basis that HI values, the non-carcinogenic risk is classed into negligible (risk level 1; HI < 0.1), low risk (risk level 2; HI ≥ 0.1 < 1), medium risk (risk level 3; HI ≥ 1 < 4), and high risk (risk level 4; HI ≥ 4) (Bortey-Sam et al. 2015; Mgbenu and Egbueri, 2019; Egbueri, 2020a).
Carcinogenic health risk assessment
The cancer risks relating to the ingestion of the water resources were evaluated as the incremental threat of a person developing cancer over a lifetime, emanating from the exposure to a potential carcinogen (Rahman et al. 2017). The probability of cancer risk (PCR) was estimated by multiplying the CDI (mg/kg/day) with the slope factor (SF) (mg/kg/day).
The acceptable value is ≤ 1 × 10−6, which means that approximately 1 per 1,000,000 will probably develop cancer as a result of the exposure to a carcinogen (Adamu et al. 2014; Ihedioha JN et al. 2021). However, risk in the range of 1 × 10−6 – 1 × 10−4 typically has been reported to be acceptable (US-EPA1999; Rahman et al. 2015). SF values for Cd are 0.38 (Egbueri et al. 2020) and Pb is 0.0085 (Patrick-Iwuanyanwu and Chioma, 2017; Naveedullah et al. 2014).
Statistical analysis
Statistical analysis was performed using Origin Pro 9. Multivariate analysis of water quality data was explored using Pearson correlation (PC) and cluster analysis (CA). Hierarchical clustering was performed to measure the similarities between the clusters. ANOVA and Tukey’s post hoc multiple comparisons were done to compare water quality parameters.
Results and discussion
The results of the physicochemical parameters analyzed for the dry and rainy season samples are presented in Table 3. The water temperature ranged from 21.73 to 30.33 °C in the dry season and 20.93–22.47 °C in the rainy season. The spring water sample temperatures were relatively low. Cold waters are generally more suitable for drinking because high water temperature enhances the growth of micro-organism and causes taste, odour, colour, and corrosion problem (Okoye et al. 2006). Temperature values obtained during the dry season in Iyi Ase, Iyi Atturu and Iyi Omu were all above ambient level (25 °C) of the Nigerian Standard for Drinking Water Quality (NSDWQ 2007) (Fig. 2). This could be as a result of the period of collection. The dry season is usually characterized by high temperatures that could be above 40 °C. However, the temperature of Iyi Ochiozo and Iyi Aguaba spring water were very low, while in the rainy season, the whole sampled locations recorded temperature below ambient level.
The pH values of the spring water were slightly acidic (4.95–5.73 in dry season and 4.23 to 4.73 in rainy season). These values were below 6.5–8.5 recommended by WHO (2017a, b), NSDWQ (2007) and EPA (2009) (Fig. 2). Water with a pH of less than 6.5 can leach metal ions like Fe, Mn, Cu, Pb and Zn from pipes and plumbing materials. Also, water with pH value above the range of 6.5–8.5 is termed secondary contaminant according to US EPA (2022). Consumption of acidic water may expose humans to some negative health effects (Egbueri et al. 2021). For example, there is an increased dissolution and absorption of heavy metals in human body under acidic conditions, thereby leading to bioavailability and bioaccumulation of heavy metals in the human system. Continuous ingestion of acidic water can lead to the erosion of enamel (which is the hard part of the tooth) and exposure of dental soft tissues. The acidic nature of the spring water could be attributed to the lithography of the study area. Nsukka FORMATION (Upper coal measures) is stratified mainly as interbedded shales, siltstone shale, sandy shale, and coal shale. Leaching of minerals from the embedded coal deposit releases acidic oxides which increase the acidity of the spring water. The relative abundance of major dissolved chemical constituents in water is dependent upon weathering process and inputs from anthropogenic activities and the type of geological rock (Singh and Hasnain 1999). The pH values obtained in this study were slightly lower than the mean value (6.8) reported by Dippong et al. (2018) in the assessment of water in the Strˆımtori-Firiza reservoir in Northwest Romania. Also, Oladipo et al. (2005) reported higher mean pH values (6.60) of spring water of Ikogosi Warm Spring in Southwestern Nigeria.
The total dissolved solids (20–92.24 mg/L) for all the five sampled locations were within the guideline values of 500 mg/L of (NSDWQ 2007, WHO 2017a, b, US EPA 2009). TDS in water indicates the presence of both organic and inorganic material in the water (Rahmanian 2015). The lower values of the TDS show that the level of saltwater incursion, human input through bunkering activities and the redox potential of the water system, which are a major contributor to higher TDS values of water, are still within the bearable limit (Epkete 2002).
The total suspended solids (TSS) were within the guideline values of 25 mg/L of (WHO 2017a, b; NSDWQ 2007) for all the sampled locations. TSS has a direct relationship with turbidity, and it ranged from 7.54 to 8.84 mg/L in the rainy season, while in the dry season, it was 6.00 – 13.33 mg/L. Seasonal variation showed that virtually, all sites have appreciable higher values of TSS during the dry season compared with that of the rainy season (p < 0.05).
The hardness of water is mainly due to dissolved alkaline earth metals, such as Ca and Mg, with other divalent cations also enhancing the hardness (Barakat et al. 2018). The rocks surrounding the water body are largely the source of total hardness (TH), although some anthropogenic activity could contribute to different concentrations (Bouslah et al. 2017). The total hardness for all the sampled locations was within the guideline values of 500 mg/L (WHO 2017a, b). There was a significant (p < 0.05), seasonal variation in the TH values. This could be traced to the solvent action of rainwater (runoff from nearby farmland) coming into contact with soil and rocks capable of dissolving Ca and Mg. This is in line with the observation of Vilane and Dlamini, (2016).
Total alkalinity (TA) values for the spring water samples ranged from 83.33 to 300 mg/L in the dry season and were undetected during the rainy season. The TA in all the sampled locations were significantly different (p < 0.05). The total alkalinity obtained from Iyi Ase, Iyi Ochiozo and Iyi Aguaba was within the limits of WHO (2017a, b) and NSDWQ (2007), while Iyi Atturu and Iyi Omu were above the guideline values of 200 mg/L. The total alkalinity in water is primarily attributed to the presence of weak acid salts, although strong bases may also contribute (OH−) in some extreme environmental conditions (Abdulwahi 2013).
The chloride values ranged from 11.92 to 21.17 mg/L in the dry season and 12.78 to 31.24 mg/L in the rainy season. These differences could be attributed to the variations in rainfall, geology, dissolution of fluid inclusions, or the existence of potentially polluted sources, fertilizers, Cl− bearing minerals at these sites, such as domestic effluent, and open grazing of cattle. The Cl− values during rainy and dry seasons were all within the guideline values (250 mg/L) for drinking water which is in line with the EPA and NSDWQ standards (EPA 2009 and NSDWQ2007). Chloride values in all the spring water were found to be significantly higher (p < 0.05) in rainy season than in dry season. This could be attributed to runoffs into groundwater and lithography of the study area. Chloride ions from water bedded coal bed, and shale coned could have been leached into the water (Taylor, 1987). The presence of higher levels of SO42− could lead to a detectable taste and may cause a laxative effect in unfamiliar consumers (WHO 2017a, b). Sulphates in the aquifer system are derived mainly from pyrite and gypsum weathering, in addition to the inputs from anthropogenic activities (Ziani et al. 2017). The SO42− values of the sampled spring water in the dry and rainy seasons ranged from 32.29 to 45.54 mg/L and 4.48 to 7.04 mg/L, respectively. The lowest value of SO42− was observed in dry season in Iyi Ase (4.48 mg/L) (Table 4). Sulphate values were within the guideline values (250 mg/L) recommended by the EPA for drinking water (EPA, 2009). The low concentration of SO42− in the rainy season reflects the dilution of groundwater by runoffs from different sources.
The electrical conductivity (EC) is a valuable measure of the amount of dissolved metal ions in water. The values of EC in all sampling points ranged from 5.46 to 7.97 μS/cm in the dry season and 4.53 to 5.70 μS/cm in the rainy season. The values obtained were within the standard values (1000 μS/cm) of NSDWQ (2007) and WHO (2017a, b) for drinking water quality. There were no significant differences (p > 0.05) in the conductivity values of all the different sampled locations in the dry and rainy season.
The NO3− concentrations in all the samples ranged from 2.57 to 5.13 mg/L in the dry season and 1.06 to 1.16 mg/L in the rainy season. The NO3− values of all the spring water samples were within the guideline value of 50 mg/L (WHO 2017a, b; NSDWQ 2007). The NO3− concentration during the rainy season was significantly lower (p < 0.05) than the concentration in the dry season.
One of the most important groups of mineral water is the alkaline waters, which are characterized by the presence, in predominating quantities, of one or more of the alkaline or alkaline earth carbonates or bicarbonates. These are the carbonates and bicarbonates of Na, Mg, Li, K and Ca. HCO3− and CO32−, which produce an alkaline environment, were observed to be within the acceptable limit (250 mg/L and 0–3 mg/L, respectively) by WHO (2017a, b) and FAO (1004) in both seasons, except for Atu spring water (300 mg/L and 3.65 mg/L, respectively). Similar results were observed in Ajali River, Nigeria (Abugu et al. 2021). Carbonates and bicarbonates are generated by the action of CO2 in water on carbonate rocks such as limestone and dolomite. Bicarbonate is of little significance in public water supplies except in large amounts, where taste is affected or where the alkalinity affects the corrosiveness of the water.
Mineral elements
A mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life (Zoroddu et al. 2019). However, the four major structural elements in the human body by weight (oxygen, hydrogen, carbon, and nitrogen), are usually not included in the list of major nutrient minerals (nitrogen is considered a “mineral” for plants, as it is often included in fertilizers). These four elements compose about 96% of the weight of the human body, and major minerals (macro-minerals) and minor minerals (also called trace elements) compose the remainder (Berdanier et al. 2013).
Table 4 presents the mean concentration of mineral elements in the spring water in both seasons. Ca and Mg are essential parameters for evaluating water quality because of their direct relationship with a hardness of water development. The concentrations of these two metals in natural water depend upon the type of rocks (Sharma and Bhattacharya 2017). They are both essential to human health in limited amounts. Magnesium is part of the chlorophyll, stimulating the nitrogen fixing algae (Roșca et al. 2020; Dippong et al. 2020). The Mg/Ca ratio exchange can cause density reduction in somezooplankton and freshwater fish species (Roșca et al. 2020; Vigh, 2008). The mean concentration of calcium ranged from 0.03 to 0.87 mg/L in dry season and 2.13 to 13.07 mg/L in rainy season (Fig. 3). The values obtained were within the standard guideline values of 75 mg/L (NSDWQ 2007; WHO 2017a, b) for drinking water quality. On the other hand, the magnesium values ranged from 0.01 to 0.43 mg/L in dry season and 1.76 to 6.08 mg/L in rainy season (Table 5). The results revealed that the values of Mg2+ at all sampled locations did not exceed the guideline values of 50 mg/L (NSDWQ 2007; WHO 2017a, b; US EPA, 2009). Okechukwu et al. (2013) obtained higher concentrations of calcium in Asho and Ajie springs (26.65 and 24.04 mg/l, respectively) in the water quality evaluation of spring waters in Nsukka, Nigeria. The variation in Ca2+ and Mg2+ levels might be related to the weathering of rocks and mineral content of each ion, such as the acidic nature of the spring water which could be attributed to the lithography of the study area. Nsukka FORMATION (Upper coal measures) is stratified mainly as interbedded shales, siltstones shale, sandy shale and coal shale. Magnesium and calcium concentrations were significantly (p < 0.05) higher during the rainy season than in the dry season in all the spring water samples. Higher concentration of calcium was observed in Iyi Ochiozo in dry season and Iyi Aguaba in rainy season, while that of magnesium was observed in Iyi Aguaba in both seasons. Generally, the concentrations of calcium were more than that of magnesium and could be attributed to the chemical properties of the soil and geological origin of spring water source in the sampled area (Toma et al. 2013).
Sodium and potassium are also widely used as essential indicators for assessing water quality. Sodium in the aquifer system is primarily derived from the dissolution of salt minerals and silicate weathering (Ziani et al. 2017). In the present study, the values of Na+ ranged from 0.17 to 0.24 mg/L in dry season and 2.35 to 2.69 mg/L in rainy season (Fig. 3). The differences in Na+ values could be related to the geological variation or rock mineral composition of the sampled locations. On the other hand, K+ values ranged from 0.98 to 2.45 mg/L in dry season and 0.23 to 0.43 mg/L in rainy season (Fig. 3). The Na+ and K+ concentrations from the sampled locations were lower than the guideline values during dry and rainy seasons. In almost all the spring water sampled, the concentrations of K+ were slightly lower than Na+. Potassium ion is fixed by clay minerals and participates in the formation of secondary minerals, and this could explain the low K+ levels (Scheytt 1997). The seasonal variation showed that Na+ concentrations in all the sampled locations were significantly higher (p < 0.05) in rainy season than dry season, while that of K+ values at all the sampled locations were significantly higher (p < 0.05) in dry season than rainy season.
Trace metals
The mean concentration of trace metals (Table 4) was compared with the local and international standards for water quality. The concentration of copper ranged from 0.01 to 0.35 mg/L in dry season and 0.04 to 0.73 mg/L in rainy season (Fig. 4). Cu concentrations in all the samples were within the guideline values of 1 mg/L (NSDWQ 2007), 2 mg/L (WHO 2017a, b) and 1.3 mg/L (US EPA 2009). Cu concentration in all the sampled location was significantly higher (p < 0.05) in rainy season than in dry season. Chemical weathering and soil leaching are the two important natural sources contributing to the increase in trace metal concentration in water (Drever 1988).
Zinc concentrations in all the spring water samples were within the guideline values of 3 mg/L (NSDWQ 2007) and 5 mg/L (US EPA 2009). However, the mean concentration of Zn in one of the springs (Iyi Ochiozo) (Table 5) was above the guideline value. In this study, the concentrations of zinc ranged from 0.03 to 6.13 mg/L in dry season and 0.03 to 0.25 mg/L in rainy season (Fig. 4). It was observed that Zn concentration in all the samples was significantly higher (p < 0.05) in dry season than in rainy season.
Cd concentrations ranged from 0.01 to 0.15 mg/L in dry season and 0.01 to 0.02 mg/L in rainy season (Fig. 4). Cadmium concentrations in all the spring water samples were above the guideline values of 0.003 mg/L (WHO 2017a, b; NSDWQ 2007) and 0.005 mg/L (US EPA 2009). The Cd concentrations were significantly different (p < 0.05) in all the samples. The high concentrations of the Cd in all the samples could be as a result of leaching of Cd from the embedded coal deposit.
Pb concentrations ranged from 0.01 to 0.05 mg/L in dry season and 0.08 to 1.08 mg/L in rainy season (Fig. 4). Lead concentrations in all the spring water samples were above the guideline values of 0.01 mg/L (NSDWQ 2007; WHO 2017a, b) and 0.015 mg/L (US EPA 2009). The high concentrations of Pb in all the samples could be as a result of leaching of minerals like lead sulphides from the embedded coal deposit.
Correlation analysis
Pearson correlation determines whether there is a linear component of association between two continuous variables and is denoted by r. Table 6 presents the correlation analysis performed on the data obtained. A strong positive and significant (p < 0.01) correlation was observed between pH and SO42− (r = 0.746), indicating that SO42− could be one of the major causes of the acidity in the spring. The relationship between pH and SO42− is in agreement with the report of Bhandari and Nayal, (2008) in Kosi River Water, Uttarakhand. The pH alternatively, strongly correlated negatively with TS, TDS, TH, NO3−, Mg and Na. Also, SO42− had strong and negative correlation with Na (r = – 0.932), NO3− (r = – 0.827), TS (r = – 0.781), TDS (r = – 0.767), and TH (r = – 0.723). There were strong positive and significant correlations at 0.01 levels for total dissolved solids and total solids (r = 0.981) indicating that TDS was the major contributing factor to TS since spring water is usually clear and has little or no suspended solids. TDS showed strong positive and significant (p < 0.01) correlations with TH, NO3−, Ca2+, Mg2+, Na+. TH similarly had a strong positive and significant (p < 0.01) correlation with Ca (r = 0.979) and Mg (r = 0.960), indicating the two ions as the source of hardness in water. This shows the influence of organic and inorganic salts in the quality of spring water (Basamba et al. 2013).
A strong positive and significant (p < 0.01) correlation was also observed between HCO3−and alkalinity (r = 0.991) and temperature (r = 0.877), showing that bicarbonate is major source of the alkalinity in water and was influenced by temperature. There were strong positive and significant correlations at 0.01 levels for Na and NO3− (r = 0.869), Zn and HO3− (r = 0.595) and Zn with total alkalinity (r = 0.582). However, weak positive and significant correlations at 0.05 level were observed for total suspended solids (TSS) and copper (r = 0.358), Pb and TSS (r = 0.380), Pb and TDS (r = 0.380), cadmium and total alkalinity (r = 0.433). EC is the measure of liquid capacity to conduct an electric charge (Marandi et al. 2013; Kumar et al. 2015). This gives an indication of the amount of total dissolved substitution in water (Yilmaz and Koc, 2014). Therefore, its ability to conduct electricity depends on dissolved ion concentrations (TDS), ionic strength, and temperature of measurements (Hem, 1985). However, the EC showed weak and insignificant relationships with the physicochemical parameters evaluated except K (r – 0.365) which was significant at p < 0.05. These results were however in contrast with the reports of Bhandari and Nayal, (2008), Basamba et al. (2013) and Oyem et al. (2014) who reported a strong correlation of EC with other parameters mostly TDS in river water, borehole and groundwater, respectively. These discrepancies could possibly be a pointer that the spring water evaluated was devoid of contamination or contains less amount of dissolved substitutions.
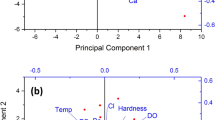
Cluster analysis
Cluster analysis (CA) is a data analysis tool used to group data having similar characteristics (Prakash and Dagaonkar 2011). The results as presented in Fig. 4 show the dendogram on hierarchical cluster analysis of the physicochemical parameters evaluated. The physicochemical parameters were grouped into four clusters. This was similar to the report of Prakash and Dagaonkar (2011) who reported that the physicochemical properties of water samples were divided into four clusters. However, there were variations in the type of parameter grouped into one cluster. This could be attributed to the differences in sample source and the concentration of the parameters in the water sample.
Cluster I comprised of pH, SO4, K and EC. This group showed that the pH of the water evaluated was strongly influenced by the concentration of SO42−, while K controls the relation between pH and SO42−. This observation was in agreement with the correlation result. In cluster II, HCO3− strongly determines the total alkalinity of the water regulated by the water temperature. More so, the concentration of Zn and Cd was slight contributors to the alkalinity of the evaluated spring water. Cluster III comprises of TS, TDS, TSS, Pb, Cu and Cl−and it showed a close relationship between TS and TDS. The total solids in the water evaluated were slightly as a result of the concentration of Pb and Cu. However, the total hardness of the water samples was as a result of the presence of Ca and Mg. This agrees with the report of Radha-Krishnan et al. (2007), Praveen et al. (2008), Oparaocha et al. (2010) and Basamba et al. (2013) that Ca as a result of anthropogenic activities, terrigenous influx in runoff, and/or natural processes within the aquifers is a major source of hardness in water. These are in agreement with the predictions by the correlation results.
Water quality index (WQI)
The water quality index estimation showed values ranging from 41.08 to 55.54 and 39.09 to 42.86 for dry and rainy seasons, respectively (Table 6). None of the spring water had excellent water status, but four of the spring’s water had good water status in both seasons. However, one spring water (Iyi Ase) had poor water status in dry season and thus is unfit for consumption. The unsuitability of the spring water samples could be due to dissolution of ions into the surface aquifer or other various anthropogenic activities within the study area such as fertilizer application, open grazing of cattle and washing of cloths. Egbueri et al. (2020) and Alum and Okoye (2020) observed similar trend in the assessment of drinking water in Ojoto area, SE, Nigeria and monitoring of pollution status of major rivers in an agricultural belt in eastern Nigeria, respectively. Nnorom et al. (2019) worked on multivariate statistical approach and water quality assessment of natural springs and other drinking water sources in Southeastern Nigeria and recorded WQI range of 3.75–70.58, 7.51–71.04, 5.81–45.92 and 1.52–132.34 for samples from springs, streams, hand-dug wells and boreholes, respectively. Paiu et al. (2014) assessed the water quality index on surface water quality in the Eastern part of Romania and got values ranging from 73.25 to 79.5, 64.25 to 60.
The spring water suitability for irrigation application
In agricultural production, water is required and is an essential resource in plants growth (Tiwari et al. 2013). Thus, spring water quality appraisal is required to determine their suitability for irrigation in Igbo-etiti farmlands. All the criteria usually used are based on the total concentrations of soluble salts, relative proportion of sodium to other cations and chemical concentration of other ions (Michael, 1992).
The PI of the spring water was calculated taking into consideration the Mg2+, Ca2+, Na+ and HCO3− concentration in the water. The prolonged use of water rich in these ions affects the permeability of the soil (Singh et al. 1999). Doneen (1964) classified irrigation based on the PI into class I, II and III. Class I and II represent 75% maximum permeability which indicates that the water sample is suitable for irrigation, while PI of 25% maximum permeability or below is seen as unsuitable for irrigation application (Doneen 1964; Raju 2007). The PI of all the analysed spring waters (Table 7) showed that they were suitable (> 75% permeability) for irrigation application.
The SSP values (Table 7) predicted that during the dry season, the spring water were not suitable (> 60%) for irrigation purposes, but showed otherwise during the rainy season (< 60%). This could be attributed to the dilution effect of rainfall on the spring water during the rainy season. SSP above 60% is not desirable in water for irrigation since it could lead to sodium accumulations which can cause a breakdown in the physical properties of the soil (Doneen 1964).
Another criterion to evaluate the suitability of water for irrigation application is the Kelly’s ratio (KR). According to KR, values less than unity (KR < 1) are suitable for irrigation, and unsuitable if KR > 1. From Table 7, both the rainy season and dry season spring water were suitable for irrigation application except Iyi Ase (KR = 4.25) during the dry season.
The magnesium adsorption ration (MAR) was also used to assess the suitability of the spring water for irrigation application. It predicted all the spring water to be within the acceptable suitability limit (MAR < 50%). MAR is based on the Mg and Ca concentration in water. According to Raghunath (1987), magnesium hazard value > 50% is detrimental to crop yield as it will make the soil more alkaline.
Chloro-alkaline indices (CAI) criterion predicted that there is no ion exchange (between K + Na with Mg and Ca), having recorded positive CAI values in all the samples. CAI gives an account of the ion exchange between the water and rock indicated.
Sodium absorption ratio (SAR) predicts sodium hazards which reduces soil permeability and inhibits water absorption by the crops (Tahmasebi et al. 2018). SAR classifies water as excellent (SAR < 10), good (SAR 10–18), doubtful (18–26) and unsuitable (SAR > 26) (Ravikumar et al. 2011). The SAR values showed excellent water for irrigation application in both seasons. Ibeneme et al. (2013) reported a similar result from the assessment of the chemical characteristics of a spring water source at Ife-Owutu, Ezinihite-Mbaise, Southeastern Nigeria.
Percent Na of the spring water samples predicted Iyi Ase, IyiAturu and Iyi Omu as unsuitable (%Na > 80) for irrigation application, Iyi Ochiozo and Iyi Aguaba as doubtful (%Na, 61 – 80) in the dry season. In rainy season, it predicted IyiOmu and Iyi Aguaba as excellent (%Na < 20), Iyi Ase as permissible and Iyi Aturu and Iyi Ochiozo as good.
Pollution index of the spring water
The pollution index (Table 8) suggested that the pollution level of all samples in dry season was insignificant (PIS < 1), while for rainy season samples, it predicted Omu and Agu to have a low pollution index (PIS > 1). Iyi Atu, Iyi Ochiozo and Iyi Ase were predicted to be heavily polluted (PIS > 2.5) which represent about 60% of the rainy season samples (Rao, 2012). This is similar to the result obtained by Egbueri et al. (2021) in underground water in Umunya area, Southeastern Nigeria. The high pollution index during the rainy season was attributed to Pb. Pb is very poisonous, even at a very low concentration (WHO 2017a, b). According to WHO (2017a, b), there is no safe Pb threshold for blood production in the body. In infants, lead can cause serious neurodevelopment problems and IQ loss. In adults, it leads to anxiety, nervous systems disorder, hypertension, kidney problems, failure of the reproductive organs, and cancer (Ukah et al. 2019; Brown & Margolis, 2012).
Health risk assessment
Spring water is one of the best sources of natural water for drinking purposes but it has been threatened by anthropogenic activities which have made some spring water unsafe for drinking. Trace metals being one of the pollutants, in this regard, were assessed for its toxicity in the spring waters. CDI, HQ, HI and PCR indices were considered, and the chronic daily intake for Pb, Cd and Cu was 0.0033, 0.0100 and 0.0233, respectively. The CDI for Cd was above the recommended daily intake of 0.003 mg/L (WHO 2017a, b), while Pb and Cu were within the limit. The HQ for Cd was greater than one across the age brackets indicating a probable non-cancer risk in the ingestion of the spring water, most especially Ase, Atu and Och spring waters in dry season and Och and Agu in rainy season (Table 9). In the rainy season samples, Pb was observed to record HQ > 1 in both adult and children. The order of non-cancer risk contribution by each metal is Cd > Pb > Cu for dry season, while Pb > Cd > Cu was the order for rainy season. These contributions led to the HI being greater than unity showing a non-cancer risk involved in the ingestion of most of the spring water in Igbo-Ettiti area of Enugu state, Nigeria. The probable cancer risk analysis of the spring waters showed a probable cancer risk, PCR value > 1 × 10–4, (Cao et al. 2014) in almost all the spring water in Igbo-Ettiti. This is inline with observation of Egbueri (2020a,b) in groundwater samples in Onitsha, Nigeria.
Conclusion
This study was conducted to assess the quality of spring water in Igbo-Etiti, Nigeria, for drinking and irrigation purposes based on several physicochemical parameters, mineral elements and trace metal analysis. The spring water was generally acidic and this was attributed to the leaching of minerals from the embedded coal deposit that releases acidic oxides. Virtually, all the physicochemical parameters were within their respective standard limits, just as the mineral elements were within the required standard in both sampled seasons. Lead and cadmium concentrations were above the guideline values of NSDWQ, WHO and US EPA and this was attributed to the leaching of minerals like lead sulphides from the embedded coal deposit. Strong correlation was observed between the parameters indicating similarity in their sources. The CA confirms that the correlation analysis was very strong within the similar group (homogeneous) which confirmed the observation made by correlation analysis. All the spring water samples had good water status except one (Iyi Ase) is considered unsafe for drinking. For irrigation application, the water quality indices and percent sodium of the spring water were generally predicted to be suitable except Iyi Ase. The pollution indices suggested that the pollution level was insignificant in both season and the probable cancer risk analysis of the spring water showed a probable cancer risk (PCR value > 1 × 10–4). These observations are in contrast to reports by Rao (2012). Therefore, it is strongly recommended that the spring water must be treated before use.
References
Abugu HO, Egwuonwu PF, Ihedioha JN, Ekere NR (2021) Hydrochemical evaluation of river Ajali water for irrigational application in agricultural farmland. Appl Water Sci 11:71. https://doi.org/10.1007/s13201-021-01395-4
Adamu CI, Nganje TN, Edet A (2014) Heavy metal contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Nanotechnology Monitoring Managment, Environmental. https://doi.org/10.1016/j.enmm.2014.11.001
Alum OL, Okoye COB, Abugu HO (2021) Quality assessment of groundwater in an agricultural belt in eastern Nigeria using a water quality index. Afr J Aquat Sci. https://doi.org/10.2989/16085914.2021.1882375
Akhtar MM, Tang Z, Mohamadi B (2014) Contamination potential assessment of potable groundwater in Lahore. Pol J Environ Stud 23(6):1095–1916
Abdulwahid S (2013) Water quality index of delizhiyan springs and Shawrawa river within Soran district, Erbil, Kurdistan region of Iraq. J Appl Environ Biol Sci 3:40–48
Akande SO, Ogunmoyero IB, Petersen HI, Nytoft HP (2007) Source rock evaluation of coals from the Lower Maastrichtian MamuFORMATION, SE Nigeria. J Pet Geol 30(4):303–324
Alum OL, Okoye COB (2020) Pollution status of major rivers in an agricultural belt in Eastern Nigeria. Environ Monit Assess 192:393. https://doi.org/10.1007/s10661-020-08366-3
Bhat SU, Mushtaq S, Qayoom U, Sabha I (2020) Water quality scenario of Kashmir Himalayan springs—a case study of Baramulla district Kashmir Valley. Water Air Soil Pollut 231:454
Bhat SU, Nisa AU, Sabha I, Mondal NC (2022) Spring water quality assessment of Anantnag district of Kashmir Himalaya: towards understanding the looming threats to spring ecosystem services. Appl Water Sci 12:180. https://doi.org/10.1007/s13201-022-01691-7
Barakat A, Meddah R, Afdali M, Touhami F (2018) Physicochemical and microbial assessment of spring water quality for drinking supply in Piedmont of Béni-Mellal Atlas (Morocco). Phys Chem Earth Parts a/b/c 104:39–46
Basamba TA, Sekabira K, Kayombo CM, Ssegawa P (2013) Application of factor and cluster analyses in the assessment of sources of contaminants in borehole water in Tanzania. Pollut J Environ Stud 22(2):337–346
Berdanier Carolyn D, Dwyer Johanna T, Heber D (2013) Handbook of Nutrition and Food (3rd ed.). CRC Press. p. 199. Retrieved 3 July 2016.
Bhandari NS, Nayal K (2008) Correlation study on physicochemical parameters and quality assessment of Kosi River Water. Uttarakhand E-Journal of Chemistry 5(2):342–346
Biswas AK, Tortajada C (2010) Future water governance: problems and perspectives. Int J Water Resour 26(2):129–139
Bortey-sam N, Nakayama SM, Ikenka Y, Akoto O, Baidoo E (2015) Human health risks from metal and metalloid via consumption of food animal near gold mines in tarkwa, Ghana: estimation of the daily intake and targeted harzard quotient (THQs). Ecotoxicol Environ Saf 111:160–167
Bouslah S, Djemili L, Houichi L (2017) Water quality index assessment of KoudiatMedouar Reservoir, northeast Algeria using weighted arithmetic index method. J Water Land Develop 35:221–228
Brown MJ, Margolis SB (2012) Lead in drinking water and human blood lead levels in the United States, Morbidity and Mortality Weekly Report (MMWR) Supplements. 61(04):1–9.
Cao S, Duan X, Zhao X, Ma J, Dong T, Huang N, Wei F (2014) Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China. Sci Total Environ 472:1001–1009. https://doi.org/10.1016/j.scitotenv.2013.11.124
Chatterji C, Raziuddin M (2002) Determination of Water Quality Index (WQI) of a degraded river in Asanol Industrial area, Rainganj, Burdwan, West Bengal. Nat Environ Pollut Technol 1(2):181–189
Chukwu KE (1999). Water supply systems and environmental health. Workshop on environment, sanitation and human existence, UNTH, Enugu, 4th-5th August, pp. 16–21.
Dahunsi SO, Owamah HI, Ayandiran TA, Oranusi US (2014) Drinking water quality and public health of selected communities in South Western Nigeria. Water Qual Expo Health 6:143–153
Doneen LD (1964) Notes on water quality in agriculture. University of California
Dippong T, Mihali C, Na ˘sui D, Z, Berinde, C, Butean (2018) Assessment of water physicochemical parameters in the Strˆ ımtori-Firiza Reservoir in Northwest Romania. Water Environ Res 90:220–233. https://doi.org/10.2175/106143017X15054988926578
Dippong T, Mihali C, Hoaghia MA, Cical E, Cosma A (2019) Chemical modeling of groundwater quality in the aquifer of seini town – someș plain, northwestern Romania. Ecotox Environ Saf 168:88–101. https://doi.org/10.1016/j.ecoenv.2018
Dippong T, Hoaghia M, Mihali C, Cical E, Calugaru M (2020) Human health risk assessment of some bottled waters from Romania. Environ Pollut 267(2020):115409. https://doi.org/10.1016/j.envpol.2020.115409
Drever JF (1988) The chemistry of natural waters. 3rd edition New York, Prentice-Hall
Ehiemere VC, Ihedioha JN, Ekere NR, Ibeto CN, Abugu HO (2022) Pollution and risk assessment of heavy metals in water, sediment and fish (Clariasgariepinus) in a fish farm cluster in Niger Delta region Nigeria. J Water Health. 5:41. https://doi.org/10.2166/wh.2022.003
Egbueri JC (2020a) Heavy metals pollution source identifcation and probabilistic health risk assessment of shallow groundwater in Onitsha. Anal Lett, Nigeria. https://doi.org/10.1080/00032719.2020.1712606
Egbueri JC (2020) Heavy metals pollution sources identification and probabilistic health risk assessment of shallow groundwater in Onitsha Nigeria. Anal Lett. https://doi.org/10.1080/00032719.2020.1712606
Egbueri JC, Mgbenu CN, Daniel Digwo C, Nnyigide CS (2021) A multi-criteria water quality evaluation for human consumption, irrigation and industrial purposes in Umunya area, southeastern Nigeria. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1907360
Ekpete OA (2002) Physicochemical determination of river water in Odhiolugboji community in Rivers State. J Vocational Sci Educ Develop 3(1):25–29
Egbueri JC, Unigwe CO (2019) An integrated indexical investigation of selected heavy metals in drinking water resources from a coastal plain aquifer in Nigeria. SN Appl Sci 5:3. https://doi.org/10.1007/s42452-019-1489-x
Egbueri JC, Ameh PD, EzugwuCK OOS (2020) Evaluating the environmental risk and suitability of hand-dug wells for drinking purposes: a rural case study from Nigeria. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1800000
Egbueri JC, AmehPD UCO (2020) Integrating entropy-weighted water quality index and multiple pollution indices towards a better understanding of drinking water quality in Ojoto area, SE Nigeria. Sci Afr. https://doi.org/10.1016/j.sciaf.2020.e00644
Egwuonwu PF, Ihedioha JN, Abugu HO (2021) Ekere NR (2021) Impact of some beverage industries on the physicochemical Characteristics of Ajali River in Enugu. Nigeria Environ Monit Assess 193:136. https://doi.org/10.1007/s10661-021-08912-7
FAO (2002) Biodrainage - Principles, Experiences and Applications. International Programme for Technology and Research in Irrigation and Drainage Food and Agriculture Organization of the United Nations. https://www.fao.org/3/Y3796E/y3796e00.htm#Contents
Goldscheider N, Chen Z, Auler AS, Bakalowicz M, Broda S, Drew D, Hartmann J, Jiang G, Moosdorf N, Stevanović Z, Veni G (2020) Global distribution of carbonate rocks and karst water resources. Hydrogeol J 28:1661–1677. https://doi.org/10.1007/s10040-020-02139-5
Hartmann AN, Goldscheider T, Wagener JL, Weiler M (2014) Karst water resources in a changing world: review of hydrological modeling approaches. Rev Geophys. https://doi.org/10.1002/2013RG000443
Hem D (1985) Study and Interpretation the Chemical of Natural of Characteristics Natural Water 3rd edition. USGS Water Supply Paper 2254, US Govt Printing Office Washington DC. Pp 66–69
Ibeneme SI, Ukiwe LN, Essien AG, Nwagbara JO, Nweze CA, Chinemelu ES, Ivonye CA (2013) Assessment of the chemical characteristics of a spring water source at Ife-Owutu, Ezinihite-Mbaise, Southeastern Nigeria. Am J Eng Res 2(10):282–290
Ifediegwu IS, Onyabor CF, Nnamani CM (2019) Geochemical evaluation of carbonate aquifers in Ngbo and environs, Ebonyi State, Southeastern. Nigeria Modell Earth Syst Environ 646:1–17. https://doi.org/10.1007/s40808-019-00646-3
Ihedioha JN, Abugu HO, Ujam OT, Ekere NR (2021) Ecological and human health risk evaluation of potential toxic metals in paddy soil, rice plants, and rice grains (Oryza sativa) of Omor Rice Field, Nigeria. Environ Monit Assess 193:620. https://doi.org/10.1007/s10661-021-09386-3
Iloeje NP (1995) New geography of Nigeria. Longman Nig Ltd.
Jones WR, Spence MJ, Bowman AW, Evers L, Molinari DA (2014) A software tool for the spatiotemporal analysis and reporting of groundwater monitoring data. Environ Model Softw 55:242–249
Kelly W (1940) Permissible composition and concentration of irrigated waters. In: Proceedings of the American Society of Civil Engineers 1940, Reston, VA, USA, January 1940: 66, pp 607–613
Kumar SK, Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl J Water Sci 5:335–343
Lesley M, Phil M (2009) Partners for Water and Sanitation Hydrogeological survey report analysis and recommendations. https://wedc-Knowledge.lboro.ac.uk/resources/pfws/NIG08a_Enugu_Hydrogeo_review_Aug09.pdf
Marandi A, Polikarpus M, Jõeleht A (2013) A new approach for describing the relationship between electrical conductivity and major anion concentration in natural waters. Appl Geochem 38:103–109
Michael AM (1992) Irrigation theory and practices. Vikash Publishing house Pvt. Ltd., pp 686–740
Naveedullah M, Hashmi Z, Yu C, Shen H, Duan D, Shen C, Lou L, Chen Y (2014) concentrations and human health risk assessment of selected heavy metals in surface water of the siling reservoir watershed in Zhejiang Province. China Pol J Environ Stud 23(3):801–811
Nigeria Industrial Standard (NIS) (2007). Nigeria Standard for Drinking Water Quality (NSDWQ, 2007). Abuja, Nigeria: Standard Organization of Nigeria (SON). Pp 1–30.
Nnorom IC, Wuzie UE, Eze SO (2019) Multivariate statistical approach and water quality assessment of natural springs and other drinking water sources in Southeastern Nigeria. Heliyon 5(2019):e01123. https://doi.org/10.1016/j.heliyon.2019.e01123
NSDWQ (2007) Nigerian Standard for Drinking Water Quality (NSDWQ). Nigerian Industrial Standard (NIS) 554:13e14. Available at https://www.unicef.org/nigeria/ng_publications_Nigerian_Standard_for_Drinking_Water_Quality.pdf
Nwoke SU, Ekere NR, Abugu HO, Ihedioha JN, Ezike CC, Odum PU, Eze SI (2021) Potential toxic elements (ptes) and the health risk impact of some dairy products marketed in nigeria eurasian. J Anal Chem 16(2):1–9
Obasi PN, Aghamelu OP, Akudinobi BEB (2013) Determination of Hydraulic conductivity of Sandstones of Ajali Formation in Uturu Area (Southeasthern Nigeria) using grain size analysis. J Nat Sci Res 3(3):49–54
Odum PU, Ekere NR, Abugu HO, Ihedioha, JN, Nwoke SU, Ezike CC, Eze SI (2021) Potential toxic elements load and their health risk assessment in vegetables grown in nsukka area of South-Eastern nigeria. Toxicol Int 28(2):103–114. https://doi.org/10.18311/ti/2021/v28i2/26074
Ofomata GEK (1978). The Nsukka Environment, Fourth Dimension Publishers. Nsukka (Nigeria). Reviewed https://www.eea.europa.eu> archived 26 Sep 2018. Diffuse Sources – European Environmental Agency.
Okoye COB, Ugwu E (2006) Quality of Borehole waters in Nsukka local government area of Enugu State. Nigeria Niger Annal Nat Sci 6(2):121–123
Oladipo AA, Oluyemi EA, Tubosun IA, Fasisi MK, Ibitoye FI (2005) Chemical examination of Ikogosi warm spring in south Western Nigeria. J Appl Sci 5:75–79. https://doi.org/10.3923/jas.2005.75.79
Okechukwu ME, Ogwo V, Onuegbu CU, Mbajiorgu CC, Ezenne GI (2013) Water quality evaluation of spring waters in Nsukka. Nigerian J Technol 32(2):233–240
Oluwasola HO, Oluoye O, Bashir SM, Odewole OA, Abugu HO, Akpomie KG, David MK, Fagorite VI, Umar MA (2021) Geochemical and health risk assessment of heavy metals concentration in soils around Oke-Ere mining area in Kogi State, Nigeria. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1862817
Oparaocha ET, Iroegbu OC, Obi RK (2010) Assessment of quality of drinking water sources in the Federal University of Technology, Owerri, Imo state, Nigeria. J Appl Biosci 32:19–24
Oyem HH, Oyem IM, Ezeweali D (2014) Temperature, pH, electrical conductivity, total dissolved solids and chemical oxygen demand of groundwater in Boji-BojiAgbor/Owa area and immediate suburbs. Res J Environ Sci 8(8):444–450
Owamah IH, Asiagwu AK, Egboh SHO, Phil-Usiayo S (2013) Drinking water quality at Isoko North communities of the Niger Delta Region Nigeria. Toxicol Environ Chem 95(7):1116–1128
Patrick-Iwuanyanwu K, Chioma NC (2017) Evaluation of heavy metals content and human health risk assessment via consumption of vegetables from selected markets in Bayelsa State Nigeria. Biochem Anal Biochem 6:332. https://doi.org/10.4172/2161-1009.1000332
Prakash MM, Dagaonkar A (2011) Application of cluster analysis to physicochemical parameters of MunjSagarTalab, Dhar (Madhya Pradesh, India). Recent Res Sci Technol 3(1):41–50
Praveen M, Saurabh A, Mudita N (2008) Assessment of physicochemical characteristics and suggested restoration measures for Pushkar Lake Ajmer Rajasthan (India). Proceedings of Taal2007: The World Lake Conference, 1518–1529
Rao SN (2012) PIG: a numerical index for dissemination of groundwater contamination zones. Hydrol Process 26(22):3344–3350. https://doi.org/10.1002/hyp.8456
Ravikumar P, Somashekar R, Angami M (2011) Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgium District, Karnataka State India. Environ Monit Assess 173:459–487
Raghunath HM (1987) Groundwater. Wiley Eastern Ltd., pp 344–369
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Padesh, South India. Environ Geol 25:1047–1074
Radha-Krishnan R, Dharmaraj K (2007) A comparative study on the physicochemical and bacterial analysis of drinking, borewell and sewage water in the three different places of Svakasi. J Environ Biol 28(1):105–113
Rahmanian N, Ali SHB, Homayoonfard M, Ali NJ, Rehan M, Sadef Y, Nizami AS (2015) Analysis of physiochemical parameters to evaluate the drinking water quality in the state of Perak Malaysia. J Chem 25:4. https://doi.org/10.1155/2015/716125
Richards LA (1954) Diagnosis and improvement of saline and alkali soils, United State Department of Agriculture: Washington, DC, USA, 78
Roșca OM, Dippong T, Mariana M, Mihalia M, Mihalescua L, Hoaghiab MA (2020) Jelea M (2020) Impact of anthropogenic activities on water quality parameters of glacial lakes from Rodnei mountains Romania. Environ Res 182:109136
Scheytt T (1997) Seasonal variations in groundwater chemistry near Lake Belau, Schleswig-Holstein, Northern Germany. Hydrogeol J 5:86–95
Science Michigan Sea Great. Nonprofit Web Design by Boxcar Studio, (2020). Retrieved on 16th march 2020 from htt://www.michigan Sea Great Home.
Singh AK, Hasnain S (1999) Environmental geochemistry of Damodar River basin, East Coast of India. Journal Environmental Geology 37:124–136
Sharma S, Bhattacharya A (2017) Drinking water contamination and treatment techniques. Appl Water Sci 7:1043–1067. https://doi.org/10.1007/s13201-016-0455-7
Tahmasebi P, Mahmudy-Gharaie MH, Ghassemzadeh F, Karouyeh AK (2018) Assessment of groundwater suitability for irrigation in a gold mine surrounding area. NE Iran Environ Earth Sci 77:766
Taylor OJ (1987). Oil Shale, Water Resources, And Valuable Minerals Of The Piceance Basin, Colorado: The Challenge And Choices Of Development, U.S. Geological Survey Professional Paper 1310
Toma J, Ahmed R, Abdulla Z (2013) Application of water quality index for assessment water quality in some bottled water Erbil City, Kurdistan Region, Iraq. J Adv Lab Res Biol 4:118–124
Tiwari AK, Singh PK, Mahato MK (2013) Chemistry of groundwater and their adverse effects on human health: a review. Indian J Health Wellbeing 4(4):923–927
United Nations-UN (2018) European global navigation satellite system and copernicus: supporting the sustainable development goals: building blocks towards the 2030 agenda. United Nations Ofce, Vienna
US-EPA (US Environmental Protection Agency) (1999) A risk assessment—multi way exposure spread sheet calculation tool. United States Environmental Protection Agency, Washington
US EPA (2022). Secondary Drinking Water Standards: Guidance for Nuisance Chemicals https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals. Accessed 30, June 2022.
US-EPA (US Environmental Protection Agency) (2011) Exposure factors handbook, 2011 edn (Final Report). EPA/600/R-09/052F.Washington DC
US EPA (2002) National Water Quality Inventory: 2000 Report. Environmental Protection Agency, Office of Water, EPA -841-R-02–001. Washington, D.C., USA.
US EPA (2009) United State Environmental Protection Agency. Drinking water contaminants. Washington, DC, USA. Available at: http: water. Epa.gov/drink/contaminants/index.cfm#list. Retrieved on 20th may 2019.
Ukah BU, Egbueri JC, Unigwe CO, Ubido OE (2019) Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area Lagos Nigeria Int J Energy. Water Res. https://doi.org/10.1007/s42108-019-00039-3
Vilane BRT, Dlamini J (2016) An assessment of the Mhlambanyoni spring water quality at Sigombeni, Swaziland. J Agric Sci Eng 2:40–45
Vigh MT, (2008) River Water Quality in the River Basin of Târnavei (In Romanian). Casa Cărții de Știință publishing house, Cluj- Napoca
Water science school (2018) Groundwater Flow and the Water Cyclehttps://www.usgs.gov/special-topics/water-science-school/science/groundwater-flow-and-water-cycle. Accessed 10 June, 2022.
WHO/UNICEF, (2015) World Health Organization (WHO) and United Nations Children's Fund (UNECEF), Progress on Sanitation And Drinking Water e 2015 Update and MDG Assessment, WHO Switzerland
Worldometer (2020) Elaboration of data by United Nations, department of economic and social affairs, population division. World population prospects: the 2019 revision (www.worldometer.info. Accessed 16/05/2020
WHO (World Health Organization)Guidelines for Drinking Water Quality, 3rd ed. (World Health Organization, Geneva, 2017a).
Wilcox LV (1955) Classification and Use of Irrigation Waters, Circular 969. USDA
WHO (2017b) Guidelines for drinking-water quality: first addendum to the fourth edition. WHO Geneva World Health Organization (WHO), Guideline for drinking water quality 4th Edition, WHO Switzerland. P.156.
Yilmaz E, Koc C (2014) Physically and chemically evaluation for the water quality criteria in a farm on Akcay. J Water Recours Protection 6:63–67
Yousif AM (2019) Recovery and then individual separation of platinum, palladium, and rhodium from spent car catalytic converters using hydrometallurgical technique followed by successive precipitation methods. J Chem. https://doi.org/10.1155/2019/2318157
Ziani D, Abderrahmane B, Boumazbeur A, Benaabidate L (2017) Water quality assessment for drinking and irrigation using major ions chemistry in the Semiarid Region: case of Djacer Spring, Algeria. Asian J Earth Sci 10:9–21
Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM (2019) The essential metals for humans: a brief overview. J Inorganic Biochem 195:120–29
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ezea, V.C., Ihedioha, J.N., Abugu, H.O. et al. A multi-criteria approach to drinking and irrigation water assessment of spring water in Igbo-Etiti, Nigeria. Appl Water Sci 12, 220 (2022). https://doi.org/10.1007/s13201-022-01747-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01747-8