Abstract
Osteoarthritis (OA) is a prevalent degenerative joint disease characterized by cartilage loss and accounts for a major source of pain and disability worldwide. However, effective strategies for cartilage repair are lacking, and patients with advanced OA usually need joint replacement. Better comprehending OA pathogenesis may lead to transformative therapeutics. Recently studies have reported that exosomes act as a new means of cell-to-cell communication by delivering multiple bioactive molecules to create a particular microenvironment that tunes cartilage behavior. Specifically, exosome cargos, such as noncoding RNAs (ncRNAs) and proteins, play a crucial role in OA progression by regulating the proliferation, apoptosis, autophagy, and inflammatory response of joint cells, rendering them promising candidates for OA monitoring and treatment. This review systematically summarizes the current insight regarding the biogenesis and function of exosomes and their potential as therapeutic tools targeting cell-to-cell communication in OA, suggesting new realms to improve OA management.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a chronic low-degree inflammatory disease mainly characterized by progressive degeneration of articular cartilage, thickening of the subchondral bone, synovial inflammation, meniscus and ligament degeneration, and osteophyte formation.1,2 The well-established risk factors for OA include age, sex, obesity, trauma, metabolism, and joint biomechanics.3,4,5 The chronic pain and dysfunction caused by OA affect over 250 million people worldwide,2 which severely reduces the life quality of individuals and represents a considerable socioeconomic burden.6 Currently, drug therapy serves as a fundamental strategy in the overall treatment of OA.7,8 Throughout the whole treatment process, most patients need short-term or long-term medication,7,8,9 including nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, and drugs for intra-articular injection (e.g., hyaluronic acid and glucocorticoid). However, the current drug treatment of OA suggested by international guidelines is merely aimed at remission of disease symptoms, without substantial interruption of the destructive process or restoration of lesioned cartilage in OA.8,9,10 As for patients with end-stage OA, joint arthroplasty surgery represents a prevalent treatment modality, although sometimes the functional outcome can be unsatisfactory. Moreover, joint replacement requires more revision surgery in the case of complications such as infection and prosthetic fracture.11,12 Therefore, it is essential to clarify the molecular mechanisms underlying OA occurrence and progression to facilitate new therapies for future clinical needs.
Joint cartilage tissue is in a complex microenvironment that contains not only chondrocytes but also a variety of non-chondrocyte types, such as adipocytes, synovial cells, mesenchymal stem cells (MSCs), endothelial cells, and immune cells.1,3,5 These cellular components can crosstalk with each other by secreting a variety of metabolic factors and inflammatory factors through paracrine, autocrine, and endocrine pathways and jointly maintain articular cartilage homeostasis, which is, however, heavily disrupted in OA. Accumulating evidence suggests that altered communication between chondrocytes and the surrounding tissues may directly or indirectly affect the progression of OA1,13 (Fig. 1). Upon exposure to risk factors that promote joint vulnerability, dysfunctional chondrocytes release excessive protease matrix-degrading enzymes, such as matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), leading to the degradation of extracellular matrix (ECM).14 These degradative products are released into the synovial fluid, where they act as damage-associated molecular patterns (DAMPs) to trigger the inflammatory response of adjacent synovial cells (e.g., synovial fibroblasts, macrophages, and mast cells). Subsequently, OA-related immune components, including proinflammatory cytokines (e.g., interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α)), growth factors (e.g., transforming growth factor-beta, (TGF-β)), chemokines and adipokines, are aggregated and further promote the activity of MMPs and ADAMTSs, initiating vicious feedback of local tissue damage and low-grade inflammation.14,15 Therefore, exploring how cells communicate within cartilage microenvironments may help to unveil the pathogenesis of OA and explore new strategies for future treatment of OA.
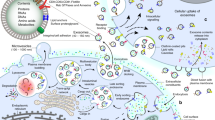
The inflammatory network and pathobiology in OA. a Risk factors for OA. Evidence suggests that specific systemic risk factors (e.g., age, obesity, and sex) and mechanical factors (e.g., trauma and joint biomechanics) are capable of causing cartilage damage in OA. b Inflammatory network in OA. The degradation products of cartilage and extracellular matrix (ECM) components are released into the joint cavity as damage-associated molecular patterns (DAMPs), which ultimately stimulate synovial cells and macrophages to secrete different types of inflammatory mediators, such as cytokines, chemokines, and complement. These molecules further lead to loss of chondrocyte phenotype and cartilage damage. Notably, the fat pad around the joints has been identified as another source of proinflammatory cytokines, especially adipokines, which may also trigger a catabolic response in chondrocytes. c Changes of osteochondral structure in OA. With the progression of OA, hypertrophy, senscent, and apoptotic chondrocytes increase, resulting in ECM catabolism as well as cracks and stratification appearing on the surface of cartilage. In the subchondral bone region, subchondral bone thickness gradually increases and calcified cartilage extends into articular cartilage, with duplication of the tidemark and invasion of vascular and nerve invasion into the cartilage region. Overall, these changes in the cartilage microenvironment can accelerate the degeneration progression of cartilage. MMPs matrix metalloproteinases, ADAMTSs a disintegrin and metalloproteinase with thrombospondin motifs, ECM extracellular matrix, DAMPs damage-associated molecular patterns, IL-1β interleukin-1beta, IL-6 interleukin-6, TNF-α tumor necrosis factor-alpha, CCL2 chemokine (C-C motif) ligand 2, CCL5 chemokine (C-C motif) ligand 5, TGF-β transforming growth factor-beta, TLR2 Toll-like receptor 2, TLR4 Toll-like receptor 4
Recently, exosomes have emerged as a new medium involved in cell–cell communication.16,17 A variety of cellular components, in particular noncoding RNAs (ncRNAs), can be preferentially capsuled into exosomes and then delivered to the surrounding or distant environment. Exosomal cargoes can regulate the biological activity of recipient cells, thus shuttling intercellular signals and triggering various physiological and pathological processes. Multiple cell lineages in the osteoarthritic joint are able to behave as active aggressors or passive responders by secreting or internalizing exosomes during disease onset and progression. Better characterization of exosomal cargoes might facilitate the identification of new indicators and inform targeted therapy for OA. In this review, we highlight the important role of exosomes in the progression of OA and discuss the prospects and challenges of their utility as clinical biomarkers or therapeutic agents for OA patients.
The biogenesis and release of exosomes
Exosomes, ranging from 40 to 160 nm in diameter,16 are lipid-bilayer extracellular vehicles (EVs) secreted by most eukaryotic cells and abundant in various body fluids, including blood, urine, saliva, breast milk, pleural effusion, and synovial fluid16,18,19,20 (Fig. 2). Typically, exosomes contain a group of common membrane and cytoplasmic proteins, including membrane transport and fusion proteins (Rab GTPases, annexin, integrin, and fibronectin),21,22 tetraspanins (CD9, CD63, CD81, and CD82),23,24 heat shock proteins (Hsp20 and Hsp27),25 and lipid-related proteins.26,27 The biogenesis of exosomes initiates with the double invagination of the plasma membrane to generate early endosomes, which subsequently maturate to intracellular multivesicular bodies (MVBs), the intermediates within the endosomal system. During this transition, exosomes, or essentially speaking, intraluminal vesicles (ILVs), are formed within the luminal space of MVBs.18,19 This process involves a continuum of mechanisms and particular sorting machinery to sequester cargoes on microdomains of the limiting membranes of MVBs, followed by inward membrane budding and fission of small vesicles harboring separated cytosol. The center of these machineries is the endosomal sorting complex required for transport (ESCRT),28,29 which acts as a driver of membrane shaping and scission of ILVs. The ESCRT machinery is mainly composed of four distinct subcomplexes known as ESCRT-0, -I, -II, and-III.28,29,30 ESCRT-0 and ESCRT-I are involved in cargo aggregation on the microdomains of MVB membranes. ESCRT-II is required for the assembly and recruitment of the ESCRT-III subcomplex, while ESCRT-III performs the budding and fission of ILVs into the MVB lumen.23 The canonical ESCRT pathway can also crosstalk with the syntein/ALIX axis to manipulate cargo clustering and membrane budding, which likely depends on the ability of ALIX to bridge the ESCRT-III subunit and cargo at the neck of ILVs.30 Notably, exosomes are still formed even when the ESCRT complex is exhausted,31 suggesting an ESCRT-independent mechanism for exosome biogenesis. Wei et al32 demonstrated that upon epidermal growth factor (EGF) stimulation, Rab GTPase 31 (RAB31) is phosphorylated by the epidermal growth factor receptor (EGFR) and further interacts with flotillin proteins in lipid raft microdomains to drive EGFR entry into ILVs in an ESCRT-independent manner. Therefore, diverse machinery can act on the endosomal system to target and package cargoes scheduled for secretion, and their involvement and contribution also shift due to cell types and the signals or stimuli that cells receive, leading to spatiotemporal dynamics of the exosomal compositional repertoire.
The processes of exosome biogenesis and release. a Biogenesis and release of exosomes. Exosome biogenesis originates from plasma membrane endocytosis, followed by maturation of early endosomes as MVBs. During this process, ILVs (exosomes) are formed within MVBs by ESCRT-dependent or ESCRT-independent pathways. In secretory cells, MVBs can be degraded by lysosomes or fused with the plasma membrane to release ILVs as exosomes with the assistance of Rab27a/b. b The contents of exosomes. Exosomes contain a variety of intrinsic components and variable cargoes, varying from proteins, lipids, metabolites, and DNAs to ncRNAs. c Interaction between exosomes and the target cells. In target cells, exosomes can directly bind with surface receptors to trigger intercellular signaling. Alternatively, exosomes can be internalized via endocytosis or direct fusion and liberate their cargoes into the target cells, thus mediating multiple physiological and pathological processes. MVBs multivesicular bodies, ILV intracavitary vesicles, ESCRT endosomal sorting complexes required for transport
Upon maturation, MVBs are basically targeted to lysosomes for degradation or alternatively transported along with microtubules to the plasma membrane for secretion, but the mechanism to balance the secretion and degradation of MVBs remains largely unknown. Once docked at the plasma membrane, the accumulated nondegradable MVBs fuse with the membrane to release ILVs as exosomes, which primarily rely on the common secretory machinery and can be promoted by several Rab family members, such as Rab27a and Rab27b.33,34 After secretion into the extracellular space, the double phospholipid membrane of exosomes protects its content from premature degradation during transportation and enables site-specific anchoring toward recipient cells.35,36 Subsequently, exosomes can interact with recipient cells in various ways, depending on cell types and the origin of exosomes.36 They can be maintained on the surface of recipient cells and directly activate surface receptors to elicit functional responses (e.g., antigen presentation). Alternatively, they can be internalized by recipient cells via direct fusion with the cell plasma membrane or via a variety of endocytic pathways, including clathrin-mediated endocytosis, micropinocytosis, phagocytosis, and caveolin- or lipid raft-mediated uptake.37,38 Following the above internalization pathways, exosomes release their contents into the cytoplasm of receptor cells and can also be degraded by lysosomes for recycling.18,35,36
Function of exosomes
The unique mechanisms governing the biogenesis of exosomes confer on them distinct sets of components, varying from proteins to nucleic acids and lipids. Their surface molecules enable physical interactions with cell populations, and their onboard cargoes initiate biological responses and phenotypic changes in recipient cells. Thus, exosomes have emerged as novel vehicles for intercellular communication, allowing the dynamic exchange of material and information between cells, tissues, and organ systems. The current interest in the field has focused on exploring the biological activity of exosomes in maintaining homeostatic balance or as a driver or consequence of pathological processes.39
Extensive studies have shown that exosomes serve as a key medium for cell–cell communication in joint cartilage tissue and are involved in the pathogenesis of OA.40,41 Cartilage-derived exosomes can impinge on the biological behavior of surrounding stromal cells, ultimately creating an appropriate microenvironment for cartilage growth.42 At the same time, stromal cells in the cartilage microenvironment can also release exosomes carrying anabolic/catabolic and anti-/proinflammatory factors, which in turn affect chondrocyte proliferation, apoptosis, and inflammatory responses, as well as the synthesis and catabolism of the cartilage ECM.41 Thus, exosome-mediated complex interactions between chondrocyte and stromal cells ensure cartilage homeostasis, while the perturbation of these processes triggers different forms of imbalances that can cause inflammation and thickening of cartilage tissue, eventually initiating or accelerating the progression of OA.
Exosomal ncRNAs in OA
Comprehensive profiling of exosomes derived from joint tissues and fluids has demonstrated that they are enriched in ncRNAs43,44 (Fig. 3), including microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs). These molecules can be preferentially loaded into exosomes and shuttled between chondrocytes or surrounding stromal cells.45,46 Moreover, the proportion of these cargoes varies depending on the donor cell types and OA staging, suggesting their properties to indicate disease status. Consequently, the focus on characterizing exosomal cargoes in OA is currently growing, and diverse exosomal ncRNAs have been documented to exhibit distinct expression profiles in the gain or maintenance of OA phenotypes.
The biogenesis and functions of miRNAs, lncRNAs, and circRNAs. a Biogenesis and functions of miRNA. After transcription by RNA polymerase II in the nucleus, primary miRNA (pri-miRNA) is cleaved by DGCR8 and the Drosha complex into precursor-miRNA (pre-miRNA). The pre-miRNA is subsequently transported from the nucleus to the cytoplasm, where it is further processed by Dicer and TRBP to a miRNA duplex. Next, the guide strand (mature miRNA) remains to form RISC protein complexes and ultimately mediates translation repression and mRNA degradation. b Biogenesis and functions of lncRNA. Several primary transcripts undergo inefficient splicing to generate lncRNAs with a 5′ cap and a 3′ poly-(A) tail structure. In the nucleus, lncRNAs are associated with chromatin remodeling and gene transcription regulation. In the cytoplasm, they can exert their function by acting as miRNA sponges, binding with proteins, or encoding regulatory peptides. c Biogenesis and functions of circRNA. circRNA forms a closed-loop structure primarily produced from pre-mRNAs via back-splicing. Based on the original sources, circRNAs can be divided into ciRNAs, EIciRNAs, and exonic ecircRNAs. ciRNAs and EIciRNAs are involved in the transcriptional modulation of their parent genes, while ecircRNAs primarily reside in the cytoplasm and can interact with miRNAs and proteins or be translatable. DGCR8 DiGeorge syndrome critical region 8, TRBP TAR RNA-binding protein, RISC RNA-induced silencing complex, ciRNAs circular intronic RNAs, EIciRNAs exon-intron circRNAs, ecircRNAs exonic circRNAs
miRNAs are endogenous small ncRNAs with a length of approximately 20–22 nucleotides. The biogenesis of miRNAs is precisely processed via a series of RNA polymerases, RNases, and RNA-binding proteins (RBPs).47,48,49 In many cases, miRNAs bind to the 3′ untranslated regions (UTRs) of messenger RNA (mRNA) to cause translational repression or target mRNA degradation.47 Abundant evidence supports the selective enrichment of miRNAs in exosomes originating from articular tissues. Importantly, exosomal miRNAs exhibit distinct patterns between OA and non-OA samples. A miRNA microarray performed on exosomes from primary chondrocytes suggested that 22 miRNAs were upregulated and 29 were downregulated in OA chondrocyte-secreted exosomes compared to the normal group.50 Another study examined exosomes from the synovial fluid of OA patients and healthy subjects,51 and the profiling of exosomal contents revealed a remarkable difference in miRNA expression, implying altered intercellular communication within the OA microenvironment.
LncRNAs are designated noncoding transcripts with a length of more than 200 nucleotides.52,53 Similar to mRNA processing, most lncRNAs are transcribed via polymerase II but probably undergo inefficient splicing.54 Based on their subcellular localization, lncRNAs can coordinate with diverse nuclear acids and proteins to regulate gene expression at multiple levels.55,56,57,58,59,60 The important roles of lncRNAs in OA progression have emerged. For example, lncRNA NEAT1 could inhibit chondrocyte proliferation and promote apoptosis by functioning as a sponge for miR-543.61 Of particular interest is the existence of certain lncRNAs in exosomes isolated from body fluids, with differential expression profiles between OA patients and healthy populations,62 indicating their preferential packaging into exosomes and potential involvement in OA evolution.
Unlike the typical linear ncRNAs, circRNAs form a closed-loop structure primarily produced from pre-mRNAs via back-splicing,63,64 endowing them with inherent resistance to exonuclease-mediated decay and thus greater stability than linear RNAs. The recent advance in RNA sequencing (RNA-seq), together with the specialized computational pipelines, has led to an explosion in circRNA characterization.65,66,67,68,69,70 Most circRNAs are predominantly distributed in the cytoplasm and execute their functions by acting as “miRNA sponges”, protein “decoys” or scaffolds, or by coding regulatory peptides.69,70,71,72 It is worth noting that circRNAs are also prevalent in various exosomes and can be transferred to recipient cells as influential messengers. The field to characterize exosomal circRNAs in OA is in infancy. A subset of exosomal circRNAs originating from human chondrocytes and MSCs actively participate in chondrogenesis and cartilage degradation.73,74 A future comprehensive illustration of exosome cargo will provide new insight into the role of circRNAs in OA pathology.
OA is a progressive joint disorder typically driven by cartilage loss/breakdown, subchondral bone remodeling, and synovial inflammation.1,2 Exosomal ncRNAs from different joint cells, such as chondrocytes,50,75 MSCs,76,77 fibroblast-like synoviocytes (FLSs),78,79 osteoclasts80 and osteoblasts,81 alter the proliferation,73,82 apoptosis,83 inflammatory response and differentiation84 abilities of target cells, thus possibly disrupting joint tissue homeostasis and affecting the development of OA (Fig. 4). In the following section, we summarize the current studies on the specific roles and mechanisms of exosomal miRNAs (Table 1), lncRNAs (Table 2), and circRNAs (Table 3) in the progression of OA.
The major biological functions of different tissue-derived exosomes in OA. a Exosomes can be released by various periarticular cells, including chondrocytes, osteoblasts, osteoclasts, and bone marrow MSCs in subchondral bone, synovial fibroblasts, macrophages, and MSCs in the synovium and infrapatellar fat pad. b Exosomes can mediate cell-to-cell communication to regulate multiple phenotypes of chondrocytes, including proliferation, apoptosis, and autophagy, thus affecting cartilage damage. c Inflammatory factors, noncoding RNAs, and other mediators released by exosomes from different cells contribute to the remodeling of the inflammatory microenvironment and drive ECM catabolism. d Exosomes and cytokines produced by osteoblasts, osteoclasts, and bone marrow MSCs in subchondral bone are transported to the cartilage layer and regulate chondrocyte metabolism. In addition, exosomes secreted by osteocytes can participate in subchondral bone remodeling, leading to changes in the biomechanical properties of OA subchondral bone, ultimately causing damage to the cartilage. OA osteoarthritis, MSCs mesenchymal stem cells, IL-1beta interleukin-1β, IL-6 interleukin-6, TNF-α tumor necrosis factor-alpha, CCL2 chemokine (C-C motif) ligand 2, TLR2 Toll-like receptor 2, TLR4 Toll-like receptor 4, MMP13 matrix metalloproteinase 13, ADAMTS5 a disintegrin and metalloproteinase with thrombospondin motifs 5, ECM extracellular matrix, MMP matrix metalloproteinase 13, SOX9 SRY-box transcription factor 9, mTOR mechanistic target of rapamycin, Wnt/β-catenin Wnt-beta-catenin, NF-κB nuclear factor kappa-B, VEGF vascular endothelial growth factor, TGF-β1 transforming growth factor-β1, IGF-1 insulin-like growth factor-1, COL2 type 2 collagen, COL10 type 10 collagen, RANKL receptor activator of NF-κB-associated phenotype β1
Exosomal ncRNAs and cartilage loss in OA
Articular cartilage loss represents the major hallmark of OA. The maintenance of cartilage integrity relies largely on the cartilage ECM, which provides the structural basis for the functional properties of articular cartilage by formulating a dense and highly organized collagenous network mainly comprised of type II collagen, as well as proteoglycans, particularly aggrecan.85 As the unique cellular component of articular cartilage, chondrocytes are solely responsible for ECM synthesis. The dynamic balance between anabolic and catabolic metabolism in the ECM is disrupted in the pathological condition of OA, where the synthesis of ECM no longer compensates for the loss of matrix integrity.14 MMPs and ADAMTS are two main families of proteinases that lead to the degradation of ECM, in particular MMP13 and ADAMTS5,86,87 which can be produced by chondrocytes or other joint cell lineages and induced by cytokines such as IL-1β and TNF-α in the extracellular space. Deletion of MMP13 and ADAMTS5 in mice can effectively control the degradation of ECM and the progression of OA degeneration.87,88 Therefore, the underlying mechanism to trigger the imbalance of ECM metabolism is multifactorial, involving dynamic crosstalk between joint cells within the cartilage microenvironment.
Chondrocytes are sparsely embedded within the ECM and constitute 2–5% of the cartilage tissue.89 Although residing in a metabolically “resting” state, chondrocytes can respond to mechanical stimuli or active biomolecules in the surrounding microenvironment in a positive or negative manner, leading to variations in the phenotype and metabolism of chondrocytes themselves and eventual changes to the composition of the ECM. Notably, the interaction between chondrocytes and their surroundings can be mediated by exosomes carrying various ncRNAs.83
Exosomal ncRNAs regulate chondrocyte proliferation in OA
Cartilage degeneration in the aged joint is tightly related to the decreased number of chondrocytes, which fail to regenerate the ECM properly. In the initial stage of OA, chondrocytes manifest transiently increased proliferation ability, which is interpreted as a repair response.89 Exosomal ncRNAs exhibit significant roles in regulating OA chondrocyte proliferation. Wang and colleagues82 investigated the potential function of exosomal miRNAs in a rat model. They found that miR-135b was upregulated in rat MSC-derived exosomes upon transforming growth factor-β1 (TGF-β1) stimulation and promoted the proliferation of rat chondrocyte C5.18 cells. Mechanistically, miR-135b negatively regulates the expression of Sp1, a transcription factor that inhibits the cell cycle by activating p15INK4b and p21WAF1/Cip1 promoters,90,91 suggesting the pro-proliferative role of MSC-derived exosomal miRNAs.
Liu and colleagues83 observed that exosomal lncRNA KLF3-AS1 derived from human MSCs induced OA chondrocyte proliferation and chondrogenic genes such as type II collagen (COL2A1) and aggrecan but suppressed the expression of MMP13 and its direct upstream transcription factor runt-related transcription factor 2 (RUNX2). The results also demonstrated that exosomal lncRNA KLF3-AS1 acted as an endogenous sponge for miR-206, thus relieving miR-206-mediated suppression of G-protein-coupled receptor kinase interacting protein-1 (GIT1), a scaffold protein that can bridge multiple protein interaction networks to promote chondrocyte proliferation.92,93 Knockdown of GIT1 significantly reversed exosomal lncRNA KLF3-AS1-mediated chondrocyte proliferation, highlighting the significance of the exosomal lncRNA KLF3-AS1/miR-206/GIT1 axis in tuning chondrocyte function and OA progression.83
Beyond miRNAs and lncRNAs, the crucial role of EV-derived circRNAs in cell proliferation has emerged. Circular RNA homologous domain interacting protein kinase 3 (circHIPK3), produced from exon 2 of the HIPK3 gene, is highly abundant in mammalian cells as well as exosomes shed by them and exhibits multifaceted roles in controlling cell growth across tissue types by sponging diverse miRNAs.94,95 Li and colleagues74 confirmed that MSC-EV-derived circHIPK3 tended to sponge miR-124-3p, a well-known antiproliferative miRNA, thus remarkably promoting chondrocyte growth and attenuating OA chondrocyte injury. These studies suggest that exosomal ncRNAs may be involved in OA development by regulating chondrocyte proliferation.
Exosomal ncRNAs regulate chondrocyte apoptosis in OA
In the late stage of OA, cartilage is characterized by hypocellularity and lacunar emptying due to excessive chondrocyte apoptosis. Chondrocyte apoptosis damages the synthesis and catabolic pathways of ECM contents, which eventually leads to ECM cartilage loss. In turn, the decrease in ECM contents further exacerbates chondrocyte apoptosis, thus entering a vicious cycle of chronic degeneration. Chondrocyte apoptosis can be triggered by multiple factors, including mechanical stress, cytokines (e.g., IL-1β), and reactive oxygen species (ROS).96 Several studies have emphasized the involvement of exosomal ncRNAs in these apoptotic pathways.97,98,99
Articular cartilage absorbs mechanical stress to stabilize the joints, constantly exposing chondrocytes to a combination of different forces. High levels of cyclic loading cause chondrocyte death and the extent of the lesion is increased with impact intensity.100 In response to cyclic loading, chondrocytes produce aberrant levels of ROS through NADPH oxidase (NOX).101,102,103 Li and colleagues97 revealed that human cord mesenchymal stem cell (hucMSC)-derived exosomal miR-100‐5p targeted NOX4 to inhibit cyclic strain‐induced ROS production and apoptosis in primary articular chondrocytes. Moreover, overexpression of NOX4 attenuated the exosomal miR-100-5p-mediated protective effects on chondrocytes. Their study preliminarily demonstrated the potential of hucMSC-derived exosomal miR-100‐5p to eliminate cyclic strain-induced chondrocyte apoptosis.
IL-1β, a proinflammatory cytokine, has been widely reported to induce chondrocyte apoptosis and cartilage destruction and is commonly used to establish in vitro models of OA. IL-1β treatment remarkably alters chondrocyte ncRNA profiles. Wang and colleagues demonstrated that IL-1β elevates lncRNA LYRM4 in chondrocytes, while exosomes originating from human bone MSCs can be taken up by chondrocytes and alleviate IL-1β-responsive LYRM4 levels and cellular apoptosis.98 However, which cargo takes control of this process needs more clarification. Similarly, circ-BRWD1 (circ_0116061) and circ_0001846 were increased in the IL-1β-treated chondrocyte cell line CHON-001 and the exosomes shed by these cells, as well as in OA cartilage tissues.73,99 Exosomal circ-BRWD1 and circ_0001846 further elevated IL-1β-induced apoptosis and MMP13 levels by eliminating the bona fide function of miR-1277 and miR-149 in CHON-001 cells, respectively. These studies suggest that exosomal ncRNAs functionally integrate into the apoptotic process of chondrocytes to modulate OA progression.
Exosomal ncRNAs regulate chondrocyte autophagy in OA
Autophagy is a highly conserved degradation system involving intracellular energy recycling among species and is generally activated by oxidative and metabolic stress.104 It serves as a protective mechanism in normal cartilage, while the compromised autophagy level favors OA-like changes in chondrocytes.105,106 With the development of OA, the expression of the mammalian target of rapamycin (mTOR), a major negative regulator of mammalian autophagy, is upregulated. A study by Wu and colleagues77 demonstrated that infrapatellar fat pad (IPFP) MSC (MSCIPFP)-derived exosomes could significantly enhance autophagy levels by inhibiting mTOR, which ultimately rescued IL-induced OA-like gene expression (MMP13 and ADAMTS5) in chondrocytes. Subsequently, exosomal RNA-seq and luciferase reporter assays revealed that miR-100-5p could target the mTOR 3’UTR among the predicted miRNAs. Intra-articular injection of antagomir-100-5p significantly demolished the remedial effect of MSCIPFP-Exos on damaged cartilage in a mouse model of surgical destabilization of the medial meniscus (DMM). Therefore, these results suggest the potential of exosomal miRNAs originating from MSCIPFP to protect articular cartilage from OA injury. However, evidence regarding the role of exosomal lncRNAs and circRNAs in OA-related autophagy is still lacking.
Exosomal ncRNAs and inflammatory microenvironment in OA
OA has been previously considered a “wear disease”.107,108 However, cumulative evidence suggests the contribution of chronic low-grade inflammation to OA symptoms and progression.14,109 Indeed, degraded ECM components or cartilage fragments accumulate in the articular cavity and are viewed as DAMPs that provoke inflammatory responses in peripheral stromal cells, including FLSs and macrophage-like synoviocytes. As a result, multiple proinflammatory factors, such as cytokines and chemokines, are released into the synovial fluid and in turn aggravate the loss of chondrocyte phenotypic stability and the degradation of ECM, causally creating a microenvironment favoring cartilage lesions. Sustained low-grade inflammation in this microenvironment alters the secretory profile of synovial tissue, particularly exosomal content.110 Currently, the engagement of exosomes in maintaining the OA inflammatory network has received extensive scrutiny.
Exosome-mediated crosstalk between FLSs and chondrocytes
FLSs, also termed synovial fibroblasts, populate the intimal lining layer of the synovial membrane. They normally secrete joint fluid that lubricates the articular cartilage. In an osteoarthritic state, however, these cells tend to express excessive MMPs and ADAMTS that tip the balance between cartilage synthesis and catabolism towards tissue destruction and behave as inflammatory aggressors through the release of exosomal cargoes, including potentially regulatory miRNAs. NanoString analysis demonstrated that the levels of 50 miRNAs differ between exosomes from IL-1β-stimulated and nonstimulated FLSs.111 FLS-derived exosomes can be readily endocytosed by chondrocytes, suggesting tight contact between these cell lineages via exosomes carrying miRNAs. miR-126-3p, a transcriptional modulator of chronic inflammation and innate immunity,112 is significantly reduced in synovial exosomes in OA patients,113 while treatment of exosomes from FLSs overexpressing miR-126-3p effectively suppresses apoptosis and inflammatory cytokine levels (IL-1β, IL-6, and TNF-α) in rat chondrocytes. Consistently, exosomal miRNA-126-3p exerts antiapoptotic and anti-inflammatory effects on articular cartilage in vivo, indicating that FLSs modulate cartilage inflammatory responses via exosomal miRNAs.
In addition to miRNAs, some exosomal lncRNAs, such as lncRNA-H1978 and lncRNA-PCGEM1,79 were involved in FLS-chondrocyte communication. H19 was significantly decreased in chondrocytes treated with IL-1β,78 whereas overexpressed exogenous H19 in FLSs can be transferred to chondrocytes through exosomes. Exosomal H19 was able to serve as a sponge for miR-106-5p, a well-characterized driver of inflammatory bone loss by targeting tissue inhibitor of metalloproteinase 2 (TIMP2),114,115 therefore weakening MMP13 and ADAMTS5 expression in chondrocytes.78 Conversely, the upregulation of lncRNA-PCGEM1 was observed in OA patient cartilage and in exosomes isolated from OA FLSs. Exosomal PCGEM1 facilitated IL-1β-induced chondrocyte apoptosis and cartilage matrix degradation.
In a persistent chronic inflammatory environment, FLSs also become passively responding effectors of exosomes shed by chondrocytes, suggesting mutual regulation between FLSs and chondrocytes to promote OA inflammation. When exosomes from IL-1β-treated chondrocytes are applied to OA synovium, a marked elevation in MMP13, IL-1β, TNF-α, and cyclooxygenase-2 (COX-2) was observed compared with nontreated exosomes.116 This potential positive feedback loop between FLSs and chondrocytes highlights the role of exosomes in contributing to the complexity of OA inflammatory networks.
Role of exosomes in macrophage-involved inflammation
FLSs are interdigitated with macrophage-like synoviocytes to constitute a delicate structure of the synovial intima. Aberrant activation or “polarization” of synovial macrophages by cartilage matrix catabolic molecules and cytokines drive synovial inflammation and OA progression.109,117,118 The polarization phenotype of macrophages varies greatly depending on the stimuli of the local environment119 (Fig. 5). For example, lipopolysaccharide (LPS), interferon-gamma (IFN-γ), or granulocyte-macrophage colony-stimulating factor (GM-CSF) can induce M0 macrophages into an M1 phenotype, producing proinflammatory cytokines (TNF-α, IL-1β, and IL-6) to elicit proinflammatory responses.118 In contrast, interleukin-4 (IL-4)/interleukin-13 (IL-13) stimulates M0 macrophages into an M2 phenotype to secrete anti-inflammatory cytokines (IL-10) and growth factors (TGF-β), ultimately suppressing inflammation and promoting tissue repair.109 In addition, M1 and M2 macrophages display distinct markers. The M1 phenotype highly expresses membrane receptor cluster differentiation 86 (CD86), toll-like receptor 4 (TLR4), and inducible nitric oxide synthase (iNOS),120 while the M2 phenotype is characterized by cluster differentiation 163 (CD163).119 The accumulation of M1-polarized macrophages in the synovial intima is the morphological characteristic of synovial inflammation, and the polarization status highly correlates with OA severity.121 In this regard, the progression of OA can be alleviated by facilitating the reprogramming of macrophages from the proinflammatory M1 to the anti-inflammatory M2 subset.122
Macrophage polarization in cartilage tissue. a The phenotype of macrophage polarization. Synovial macrophages can be derived from monocytes that originally reside in synovial tissue or in the circulation. Evidence suggests that primary macrophages (M0) can be activated and differentiate into the M1 phenotype (INF-γ and LPS stimulation) and M2 phenotype (IL-4 and IL-13 stimulation). Both M1-polarized (subdivided into M1a-c, which promotes the inflammatory response) and M2-polarized (subdivided into M2a-d, which suppresses the inflammatory response) macrophages can regulate the function of cartilage tissue through the secretion of exosomes carrying specific cytokines and chemicals. The conversion of the M2 phenotype into the M1 phenotype can effectively delay OA degeneration. b The role of exosomes in regulating macrophage polarization in OA. Left: TGF-β1-stimulated BMSC-derived exosomes attenuate cartilage injury by promoting M2 polarization of synovial macrophages through the miR-135b/MAPK6 signaling pathway. Right: Upon IL-4 or IL-13 stimulation, lncRNA MM2P is upregulated in rat macrophage (RAW264.7) cells and blocks SHP2-mediated dephosphorylation of STAT3 at Try705, thus promoting the transcription of the SOX9 gene. Alternatively, MM2P interacts with the RNA-binding protein FUS to stabilize SOX9 mRNA. Consequently, the elevated SOX9 mRNA and proteins can be delivered to chondrocytes via exosomes to promote cartilage repair. INF-γ interferon-gamma, LPS lipopolysaccharide, IL-4 interleukin (IL)-4, IL-13 interleukin (IL)-13, TGF-β1 transforming growth factor-beta1, BMSCs bone marrow mesenchymal stem cells, MAPK6 mitogen-activated protein kinase 6, SOX9 SRY-box transcription factor 9, STAT3 signal transducer and activator of transcription 3, ncRNAs noncoding RNAs, OA osteoarthritis
The involvement of exosomes in determining macrophage phenotype and behavior in the inflamed OA joint has been highlighted. Exosomes isolated from TGF-β-treated bone MSCs increased the percentage of CD163-positive macrophages (M2) in OA rat tissues. Meanwhile, these exosomes guide the phenotypic switch of LPS-primed macrophages from M1 to M2. Among the exosomal contents, miR-135b was highly expressed and probably actively involved in M2 transition by targeting mitogen-activated protein kinase 6 (MAPK6), thereby alleviating cartilage damage.123 Exosomes are also linked to the inflammatory behavior of polarized macrophages. Upon the stimulation of exosomes isolated from the synovial fluid of OA patients, GM-CSF-primed M1 macrophages release abundant IL-1β and chemokines,124 indicating that exosomes in synovial fluid serve as a key mediator to create the inflammatory microenvironment in OA. Another study75 found that exosome-like vesicles originating from OA chondrocytes can be internalized by LPS-primed macrophages and further enhance inflammasome activation and subsequent IL-1β processing. Mechanistically, these vesicles likely inhibit autophagy-related 4B (ATG4B) expression by delivering its upstream modulator miR-449a-5p into target macrophages. Subsequently, the decreased autophagy promoted the production of mitochondrial ROS, a potential signal to promote IL-1β production, suggesting the proinflammatory role of exosomes released from OA chondrocytes. Instead, exosomes from normal chondrocytes restore mitochondrial dysfunction and trigger the macrophage response towards the M2 phenotype.
Alternatively, macrophages can modulate chondrocyte behavior via exosomes. Upon IL-4 or IL-13 treatment, RAW264.7 murine macrophages and mouse bone marrow-derived macrophages undergo M2 polarization, with a dramatic increase in lncRNA MM2P.125 MM2P upregulates the expression of SRY-box transcription factor 9 (SOX9) in M2 macrophages either by interacting with the RNA-binding protein FUS to stabilize SOX9 mRNA or by blocking SHP2-mediated dephosphorylation of signal transducer and activator of transcription 3 (STAT3), which promotes SOX9 transcription. The subsequent elevated SOX9 mRNA and protein can be packaged into exosomes and transferred to mouse chondrocytes, where they can promote the secretion of ECM components. Collectively, mutual crosstalk between macrophages and other cell populations is largely attributed to exosomes released from them.
Exosomal ncRNAs and subchondral bone remodeling in OA
The articular cartilage, calcified cartilage, and subchondral bone constitute osteochondral units that work in concert to support the functional loading and motion of the joint, while progressive heterogeneous changes in the osteochondral tissues occur during OA evolution.126 ECM loss in the OA cartilage layer reduces its ability to absorb mechanical pressure and leads to excessive loading on the subchondral bone. Gradually, however, the fine balance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation is perturbed, bringing about heterogeneity in the subchondral density or stiffness that creates local shear forces to intensify cartilage deformation and further damage.127,128 Hence, OA is no longer considered a simple subject bothering articular cartilage but rather an entire joint disorder is concurrently driven by subchondral bone remodeling and subsequent neovascular invasion into the synovium and articular cartilage.129
During abnormal subchondral bone remodeling, the formation of microcracks, combined with abnormal vascularization, facilitates molecular transport between cartilage and subchondral bone. In view of this, the crosstalk between the subchondral and cartilage layers may be elevated in OA.130 Subchondral osteocytes can induce phenotypic changes in OA chondrocytes.131 This interaction might be conducted in an exosome-dependent fashion.132,133 For example, Wu et al isolated and purified exosomes from osteoblasts in the non-sclerotic or sclerotic regions of human OA subchondral bone. After co-culture with chondrocytes, exosomes from sclerotic osteoblasts triggered the expression of chondrocyte catabolism genes and reduced the expression of chondrocyte-specific markers. RNA-seq confirmed the high enrichment of miR-210-5p in the exosomes of OA sclerotic osteoblasts. Exosomal miR-210-5p increased hypertrophic and ECM-degradative gene expression and inhibited cellular aerobic respiration in chondrocytes, a feature frequently observed in OA conditions.134 Another study demonstrated a compelling case of pathological exosomal miRNA communication between osteoclasts and neighboring chondrocytes in OA.135 Following anterior cruciate ligament transection (ACLT) surgery to establish a mouse OA model, a set of miRNAs (miR-21a-5p, miR-214-3p, miR-30a-5p, and miR-30d-5p) were elevated in bone marrow osteoclasts, as well as in exosomes shed from them. Nevertheless, ablating Dicer, which is essential for miRNA biogenesis, or silencing Rab27a, a GTPase that controls exosome secretion, remarkably delayed OA progression. Strikingly, upon intravenous injection of osteoclast-derived exosomes, the preferential homing of these vesicles to chondrocytes was observed in ALCT cartilage, supporting the presumption that osteoclast-derived exosomes may pass through microcracks and vascular channels to cartilage during OA evolution. Among the exosomal contents, miR-214 was demonstrated to blunt TIMP2/3, the endogenous antagonists of MMPs and ADAMTS, consequently promoting ECM catabolism and cartilage degeneration. These studies suggest that the connection between chondrocytes and subchondral osteocytes is enhanced by exosomal miRNAs, which appear to accelerate OA progression.
Exosomal proteins in OA
Previous proteomic studies have found that proteins in exosomes are involved in OA progression.136,137 For example, Zheng and colleagues138 analyzed the protein composition of exosomes from mouse primary normal chondrocytes (D0) and IL-1β-treated chondrocytes (D3) and identified 2409 and 2077 proteins in the D0 and D3 exosomes, respectively. Subsequently, Ingenuity Pathway Analysis (IPA) and Gene Ontology (GO) analysis were performed among these identified proteins and revealed that the proteomic pattern of D3 exosomes was similar to that of D0 exosomes. However, the number of identified mitochondrial proteins was decreased in D3 exosomes, indicating the damaged chondrocyte mitochondria upon IL-1β stimulation. More importantly, intra-articular administration of D0 exosomes restored mitochondrial dysfunction and polarized the macrophage response to the M2 phenotype, successfully preventing the development of OA in mice, suggesting a potential role of chondrocyte exosomal proteins in tuning mitochondrial function. Gao and colleagues139 demonstrated that exosomes derived from knee joint fluid of osteoarthritis patients expressed significantly higher levels of inflammatory cytokines (e.g., IL-1β, TNF-α, and IFN-γ) in end-stage OA patients than in early-stage OA patients, which favored inflammation and cartilage degeneration during OA progression. In addition, Zhang and colleagues140 reported that exosomes from human embryonic stem cell-derived MSCs were able to express CD73, which can efficiently activate AKT and ERK signaling to enhance chondrocyte proliferation and infiltration, while the administration of adenosine 5′-(α,β-methylene) diphosphate (AMPCP), a CD73 inhibitor and theophylline, an adenosine receptor antagonist, effectively reduced MSC exosome-induced AKT and ERK phosphorylation in chondrocytes, highlighting the key role of exosomal CD73/adenosine axis during exosome-mediated cartilage repair. To date, there is limited evidence characterizing OA-associated exosomal proteins and the role of exosomal proteins in OA can be further explored.
Clinical significance of exosomes in OA
Exosomal cargoes as potential biomarkers for OA
Currently, X-ray141 and magnetic resonance imaging (MRI)142 are the so-called “gold standard” for OA diagnosis. When imaging signs occur, however, the degeneration of articular cartilage has often progressed to the middle and late stages that require active intervention and treatment. Although previous studies have shown the potential of matrix metalloproteinases (MMPs), lipopolysaccharide-binding protein (LBP), soluble toll-like receptor 4 (sTLR4), and urinary C-telopeptide of type II collagen (uCTX-II) as biomarkers for OA,143,144,145 but further work is required to validate their effectiveness. Hence, OA is now acknowledged as a whole joint disorder featuring heterogeneous variations in articular tissues, but the current strategies to detect such changes are unavailable due to the lack of reliable markers that indicate early changes and ongoing disease pathophysiology over time or reflect the relative involvement among individual joint tissues.
Exosomes carrying a wide range of ncRNAs have been emphasized as potential biomarkers in a variety of diseases,146,147,148,149,150 benefiting from their high abundance in body fluids,151,152 as well as the unique expression signatures of their cargoes as the disease evolves. Of note, the double-lipid membrane protects their cargo from enzyme-mediated degradation or clearance by immune systems, endowing them with an extended half-life compared with the free molecules and prompting the attention to evaluate their clinical feasibility for given diseases.137 In OA joint disease, the disruption of synovial tissue homeostasis results in an altered secretory exosome profile, which could potentially reflect the complexity and heterogeneity of the disease. Kolhe and colleagues43 characterized exosomal miRNAs in synovial fluids from OA patients and showed that the miRNA profiles differed between male and female OA patients. Certain miRNAs specific to female OA patients are relevant to the estrogen response and Toll-like receptor (TLR) signaling. For example, miR-26a, an inflammatory suppressor that targets Toll-like receptor-3 (TLR3) to ameliorate arthritis severity,153 can be induced by estrogen in FLSs, while its downregulation is observed in exosomes from female OA patients, which possibly provides hints to explain the increased prevalence and severity of OA in females than in males. Meanwhile, their team also illustrated sex-specific differences in exosomal protein cargo in synovial fluid from OA patients.154 These findings may contribute to the development of exosomal cargo-guided sex-specific monitoring for OA.
Synovial fluid-derived exosomes also vary in their contents in different OA stages. A recent study155 suggested the value of synovial fluid-derived exosomal lncRNA-PCGEM1 in discriminating the early and advanced stages of OA. Exosomal PCGEM1 in synovial fluid was significantly elevated in late OA compared with early OA, and its potential to distinguish early and late OA has been suggested based on the area under the receiver operating characteristic curve (AUC).
Circulating ncRNAs, especially miRNAs that mark early- and advanced-stage OA, have been evidenced.156,157 However, the primary concern regarding the various types of circulating biomarkers is their specificity solely for OA or at least their ability to reflect the main characteristics of cartilage degeneration. Comprehensive investigations on the clinical significance of circulating exosomal ncRNAs in the diagnosis and tracking staging of OA are still lacking. Future studies may aim to address the feasibility of examining joint tissue-derived exosomes in blood samples and discriminating the cellular origin of exosomes, which may open up the possibility to sense tissue-specific alterations as indicators for OA in a minimally invasive manner.
Potential therapeutic strategies based on exosomes
Improved understanding of exosomes in OA pathophysiology enables the development of potential therapeutic approaches to block or limit joint structure destruction. Exosome-based vehicle delivery systems and tissue engineering techniques represent advanced therapeutic strategies that have recently attracted increasing attention (Fig. 6).
The potential clinical application of exosomes in OA. a Isolation and analysis of exosomes. Pipeline of exosome separation, purification, and identification. b Exosomal contents for biomarkers. Exosomes are rich in bioactive molecules, including DNAs, mRNAs, ncRNAs, proteins, metabolites, cytokines, and lipids, and can be used as potential biomarkers; c Exosomal ncRNAs as biomarkers for OA. Exosomal miRNA and lncRNA can be used for diagnosis, prognosis, and monitoring of OA. d Exosomes act as a drug delivery system in OA. Exosomes are isolated from naive or modified donor cells to promote the efficiency of cargo delivery. e Exosome-based tissue engineering strategy to repair cartilage. Exosomes engineered for cartilage repair, combined with hydrogel scaffolding and 3D printing techniques, effectively promote in situ repair of cartilage defects. OA osteoarthritis, ECM extracellular matrix
As a promising therapeutic delivery system in OA therapy
MSCs exhibit excellent performance in tissue repair and regeneration and serve as promising seed cells in cartilage engineering approaches. These advantages are at least partially based on the paracrine effects of cellular exosomes that carry various ncRNAs to promote proliferation and inhibit inflammation in recipient cells. Therefore, MSC-derived exosomes have been considered a promising delivery system for OA therapy.
Recently, several studies have attempted to use naive MSC-derived exosomes to treat OA in vivo and in vitro models. Two individual studies demonstrated that bone marrow MSC-derived exosomes display a beneficial therapeutic effect on OA by transferring regulatory miRNAs to cartilage and balancing the synthesis and degradation of ECM.158,159 However, they focused on different miRNAs in bone marrow MSC-derived exosomes, and which cargo dominates the delay of cartilage degeneration remains unclear. To address this, some studies manipulate donor MSCs by virtue of transfection with genes of interest. miR-140, one of the most important miRNAs in chondrocytes, plays a central role in both cartilage development and homeostasis.160 Tao et al.76 overexpressed miR-140-5p in synovial MSCs using a lentiviral system and showed consistently elevated levels of miR-140-5p in the exosomes shed by these cells. Compared with non-modified exosomes, miR-140-5p-abundant exosomes showed an enhanced ability to trigger chondrocyte proliferation and migration without hindering ECM secretion in vitro and successfully prevented cartilage degeneration in OA rat models. Similarly, transfection of dental pulp stem cells (DPSCs) with miR-140-5p mimics enriches miR-140-5p in their exosomes, and administration of these exosomes remarkably improves joint conditions in a rat OA model.161
In addition to the genetic manipulation of exosomal cargoes, targeted modification of exosomes also serves as a promising OA treatment approach to deliver cargoes into chondrocytes across the dense and nonvascular ECM.162 To this end, dendritic cells are transfected with plasmids encoding fusing protein of chondrocyte-affinity peptide (CAP) and lysosome-associated membrane glycoprotein 2b (Lamp2b), which allows the release of exosomes displaying CAP-Lamp2b on the surface. The engineered exosomes are subsequently loaded with miR-140 via electroporation and are capable of transferring miR-140 into deep cartilage regions, thus alleviating OA progression in a rat model.162 These findings may allow targeted regulation of intercellular communication and point toward a potential cell-free therapy of OA alternatives to cell-based MSC therapy.
Certainly enhanced cargo delivery conducted by exosomes hinders cartilage homeostasis, which may be blocked in OA therapy. Nevertheless, the biogenesis of exosomes relies on normal cellular processes. Disrupting their activity in specific cell types or tissues remains challenging. Indeed, preferential suppression of exosomal ncRNA crosstalk to manipulate physiological or pathological processes has not yet been evidenced in vivo.
Tissue engineering strategies based on exosomes in OA therapy
Although evidence of penetrance of exosomal cargoes into cartilage is compelling, their persistence and efficacy must be carefully examined to optimize clinical potency. Tissue engineering techniques, such as hydrogels and scaffolds, have been used to better locate exosomes for exogenous drug delivery at specific cartilage defects. With these supporting materials, the retention of exosome cargo at locally defective sites can be increased to improve therapeutic efficacy.163,164 Liu and colleagues163 developed a photoinduced imine crosslinking hydrogel glue as an exosome scaffold to prepare an acellular tissue patch (EHG) for cartilage regeneration. The results showed that the EHG tissue patch could integrate seamlessly with natural cartilage, effectively preserving exosomes at defective sites and promoting cartilage repair and regeneration. Recently, three-dimensional (3D) printing technology has also been applied to the treatment of OA. Chen and colleagues164 designed a bioscaffold for MSC exosome delivery and fabricated a 3D printed ECM/gelatin methacrylate (GelMA)/exosome scaffold, which effectively restored chondrocyte homeostasis, polarized synovial macrophages to the M2 phenotype, and significantly promoted cartilage regeneration in animal models. Such properties add the possibility of exosomes for utility within regenerative therapies for OA.
Conclusions and prospects
Exosome represents a novel mode of cell-to-cell communication in joint tissues. Exosomal contents, especially ncRNAs, are selectively packaged and transferred to recipient cells to regulate their biological behaviors. Certain exosomal ncRNAs showed different expression patterns between the synovial fluid of OA patients and healthy controls, making them signs of disease onset and promising biomarkers that may inform inflammation types and disease status. Accumulating evidence also highlights novel therapeutic strategies based on exosomal ncRNAs, some of which have shown promising outcomes in vivo.
Despite the recent progress made in characterizing exosomes in OA, some challenges remain unresolved in this field, particularly in terms of the identification of the cellular origin of exosomes. Indeed, exosomes in body fluids are often heterogeneous vesicles of various origins, making it difficult to identify and isolate joint cell-specific exosomes in OA samples. Next, the involvement of exosomes in cell-to-cell communication is achieved by the delivery of specific bioactive molecules. However, how these molecules are selectively packaged into exosomes is unclear, and further studies are needed to reveal detailed mechanisms underlying exosome biogenesis. To date, only a small fraction of exosomal cargo has been identified in OA, most of which are ncRNAs. How other exosomal contents function in OA requires further investigation.
OA pathology is marked by abnormal regulation of gene expression in multiple ways. Nevertheless, a considerable portion of exosomal studies have focused merely on cartilage, synovium, or subchondral bone in isolation, ignoring the fact that OA is a whole joint disorder. In the case of viewing these in vitro studies, however, caution must be taken in regard to the pathophysiological correlation, such as whether the stimuli can pathologically mimic the inducers of arthritis. Additionally, the field to examine the therapeutic potential of exosomal ncRNAs is still in its infancy. Current studies confirm that exosomes can be engineered to deliver therapeutic factors to recipient cells for therapeutic applications, but one premise is that exosomes must be able to accurately find their targets and release their cargo to target the specificity of tissues such as chondrocytes and synovial cells. Hence, more studies are needed to identify specific markers on the membrane of joint cell populations. Accordingly, exosomes with surface modifications are designed to identify these markers, enabling targeted intervention of intercellular crosstalk. Moreover, OA is a chronic degenerative disease and the progression of OA can be controlled using “stepped therapy” at different stages. Therefore, how to accurately subtype OA by exosomes, especially in the early stage of OA, is crucial for the clinical treatment of OA. In addition, previous studies have evaluated the feasibility of sampling patient blood or synovial fluid to isolate exosomes for OA diagnosis. Non-invasive methods for exosome acquisition from other body fluids (e.g., urine, saliva) are expected to relieve the pain of patients. With an increasing exploration of these unknowns, we believe that exosomes will become feasible in the future diagnosis and treatment of OA.
References
Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072 (2016).
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet 393, 1745–1759 (2019).
Guilak, F., Nims, R. J., Dicks, A., Wu, C. L. & Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 71-72, 40–50 (2018).
Katz, J. N., Arant, K. R. & Loeser, R. F. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325, 568–578 (2021).
Chen, D. et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 5, 16044 (2017).
Collaborators, G. B. D. R. F. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1923–1994 (2018).
Roos, E. M. & Arden, N. K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 12, 92–101 (2016).
Bannuru, R. R. et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 27, 1578–1589 (2019).
Latourte, A., Kloppenburg, M. & Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 16, 673–688 (2020).
Steinert, A. F. et al. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. Ther. 9, 213 (2007).
Bendich, I. et al. Antibiotic-laden bone cement use and revision risk after primary total knee arthroplasty in US veterans. J. Bone Jt. Surg. Am. Vol. 102, 1939–1947 (2020).
van Schie, P., van Steenbergen, L. N., van Bodegom-Vos, L., Nelissen, R. & Marang-van de Mheen, P. J. Between-hospital variation in revision rates after total hip and knee arthroplasty in the Netherlands: directing quality-improvement initiatives. J. Bone Jt. Surg. Am. 102, 315–324 (2020).
Zhou, Q., Cai, Y., Jiang, Y. & Lin, X. Exosomes in osteoarthritis and cartilage injury: advanced development and potential therapeutic strategies. Int J. Biol. Sci. 16, 1811–1820 (2020).
Liu-Bryan, R. & Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 11, 35–44 (2015).
Loeser, R. F., Goldring, S. R., Scanzello, C. R. & Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707 (2012).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, 640 (2020).
Tkach, M. & Thery, C. Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232 (2016).
Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 28, R435–R444 (2018).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Garikipati, V. N. S., Shoja-Taheri, F., Davis, M. E. & Kishore, R. Extracellular vesicles and the application of system biology and computational modeling in cardiac repair. Circ. Res. 123, 188–204 (2018).
Fan, S. J. et al. Glutamine deprivation alters the origin and function of cancer cell exosomes. EMBO J. 39, e103009 (2020).
Liu, S. et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res. Cardiol. 115, 22 (2020).
Larios, J., Mercier, V., Roux, A. & Gruenberg, J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 219, e201904113 (2020).
Tobon-Arroyave, S. I., Celis-Mejia, N., Cordoba-Hidalgo, M. P. & Isaza-Guzman, D. M. Decreased salivary concentration of CD9 and CD81 exosome-related tetraspanins may be associated with the periodontal clinical status. J. Clin. Periodontol. 46, 470–480 (2019).
Reddy, V. S., Madala, S. K., Trinath, J. & Reddy, G. B. Extracellular small heat shock proteins: exosomal biogenesis and function. Cell Stress Chaperones 23, 441–454 (2018).
Paolicelli, R. C., Bergamini, G. & Rajendran, L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405, 148–157 (2019).
Safdar, A., Saleem, A. & Tarnopolsky, M. A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 12, 504–517 (2016).
Schoneberg, J., Lee, I. H., Iwasa, J. H. & Hurley, J. H. Reverse-topology membrane scission by the ESCRT proteins. Nat. Rev. Mol. Cell Biol. 18, 5–17 (2017).
Vietri, M., Radulovic, M. & Stenmark, H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 21, 25–42 (2020).
Baietti, M. F. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677–685 (2012).
Stuffers, S., Wegner, C. S., Stenmark, H. & Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10, 925–937 (2009).
Wei, D. H. et al. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 31, 157–177 (2021).
Ostrowski, M. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–U61 (2010).
Song, L. et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat. Commun. 10, 1639 (2019).
O’Brien, K., Breyne, K., Ughetto, S., Laurent, L. C. & Breakefield, X. O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 21, 585–606 (2020).
Raimondo, F., Morosi, L., Chinello, C., Magni, F. & Pitto, M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics 11, 709–720 (2011).
Mulcahy, L. A., Pink, R. C. & Carter, D. R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell Vesicles 3 (2014).
Parada, N., Romero-Trujillo, A., Georges, N. & Alcayaga-Miranda, F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J. Adv. Res. 31, 61–74 (2021).
Huang, Y. et al. Influence of species and processing parameters on recovery and content of brain tissue-derived extracellular vesicles. J. Extracellular Vesicles 9, 1785746 (2020).
Toh, W. S., Lai, R. C., Hui, J. H. P. & Lim, S. K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 67, 56–64 (2017).
Asghar, S., Litherland, G. J., Lockhart, J. C., Goodyear, C. S. & Crilly, A. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology 59, 57–68 (2020).
Di Nicola, V. Degenerative osteoarthritis a reversible chronic disease. Regen. Ther. 15, 149–160 (2020).
Kolhe, R. et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 7, 2029 (2017).
Xie, F. et al. Role of microRNA, LncRNA, and exosomes in the progression of osteoarthritis: a review of recent literature. Orthop. Surg. 12, 708–716 (2020).
Lin, Z. et al. Selective enrichment of microRNAs in extracellular matrix vesicles produced by growth plate chondrocytes. Bone 88, 47–55 (2016).
Mitton, E., Gohr, C. M., McNally, M. T. & Rosenthal, A. K. Articular cartilage vesicles contain RNA. Biochem Biophys. Res. Commun. 388, 533–538 (2009).
Peng, Y. & Croce, C. M. The role of MicroRNAs in human cancer. Signal Transduct. Target Ther. 1, 15004 (2016).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Ha, M. & Kim, V. N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 15, 509–524 (2014).
Mao, G. P. et al. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J. Cell. Mol. Med. 22, 5354–5366 (2018).
Withrow, J. et al. Synovial fluid exosomal miRNA profiling of osteoarthritis patients and identification of synoviocyte-chondrocyte communication pathway. In ORS 2016 Annual Meeting, Poster 1350 (2016).
Ransohoff, J. D., Wei, Y. & Khavari, P. A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 19, 143–157 (2018).
Quinn, J. J. & Chang, H. Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 17, 47–62 (2016).
Statello, L., Guo, C. J., Chen, L. L. & Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118 (2021).
Chen, L. L. Linking long noncoding RNA localization and function. Trends Biochem. Sci. 41, 761–772 (2016).
Tsai, M. C. et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 (2010).
Tripathi, V. et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 39, 925–938 (2010).
Gong, C. G. & Maquat, L. E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3‘ UTRs via Alu elements. Nature 470, 284 (2011).
Du, Z. et al. Integrative analyses reveal a long noncoding RNA-mediated sponge regulatory network in prostate cancer. Nat. Commun. 7, 10982 (2016).
Kopp, F. & Mendell, J. T. Functional classification and experimental dissection of long noncoding RNAs. Cell 172, 393–407 (2018).
Xiao, P. et al. LncRNA NEAT1 regulates chondrocyte proliferation and apoptosis via targeting miR-543/PLA2G4A axis. Hum. Cell 34, 60–75 (2021).
Meng, Y., Qiu, S. Q., Sun, L. & Zuo, J. L. Knockdown of exosome-mediated lnc-PVT1 alleviates lipopolysaccharide-induced osteoarthritis progression by mediating the HMGB1/TLR4/NF-kappa B pathway via miR-93-5p. Mol. Med. Rep. 22, 5313–5325 (2020).
Li, J., Sun, D., Pu, W., Wang, J. & Peng, Y. Circular RNAs in cancer: biogenesis, function, and clinical significance. Trends Cancer 6, 319–336 (2020).
Cocquerelle, C., Mascrez, B., Hetuin, D. & Bailleul, B. Missplicing yields circular Rna molecules. FASEB J. 7, 155–160 (1993).
Zhang, J. et al. Comprehensive profiling of circular RNAs with nanopore sequencing and CIRI-long. Nat. Biotechnol. 39, 836–845 (2021).
Wu, Y. et al. The therapeutic potential and role of miRNA, lncRNA, and circRNA in osteoarthritis. Curr. Gene Ther. 19, 255–263 (2019).
Li, Z. Y. et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 22, 256–264 (2015).
Zhang, Y. et al. Circular intronic long noncoding RNAs. Mol. Cell 51, 792–806 (2013).
Memczak, S. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013).
Hansen, T. B. et al. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013).
van Heesch, S. et al. The translational landscape of the human heart. Cell 178, 242–260.e229 (2019).
Li, Q. et al. CircACC1 regulates assembly and activation of AMPK complex under metabolic stress. Cell Metab. 30, 157–173 e157 (2019).
Guo, Z. Y. et al. Exosomal circ-BRWD1 contributes to osteoarthritis development through the modulation of miR-1277/TRAF6 axis. Arthritis Res. Ther. 23, 159 (2021).
Li, S., Liu, J., Liu, S., Jiao, W. & Wang, X. Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J. Nanobiotechnology 19, 194 (2021).
Ni, Z. et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1beta production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 10, 522 (2019).
Tao, S. C. et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7, 180–195 (2017).
Wu, J. Y. et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 206, 87–100 (2019).
Tan, F., Wang, D. & Yuan, Z. The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 axis. Inflammation 43, 1498–1509 (2020).
Zeng, G. X., Deng, G., Xiao, S. L. & Li, F. Fibroblast-like synoviocytes-derived exosomal PCGEM1 accelerates IL-1 beta-induced apoptosis and cartilage matrix degradation by miR-142-5p/RUNX2 in chondrocytes. Immunol. Invest. 1–18 (2021).
Dai, J. et al. Osteoclast-derived exosomal let-7a-5p targets Smad2 to promote the hypertrophic differentiation of chondrocytes. Am. J. Physiol. Cell Physiol. (2020).
Cui, Y., Luan, J., Li, H., Zhou, X. & Han, J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 590, 185–192 (2016).
Wang, R., Xu, B. & Xu, H. G. TGF-beta 1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 17, 2756–2765 (2018).
Liu, Y. et al. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 17, 2411–2422 (2018).
Mao, G. P. et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 9, 247 (2018).
Mouw, J. K., Ou, G. Q. & Weaver, V. M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 15, 771–785 (2014).
Latourte, A. et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 76, 748–755 (2017).
Glasson, S. S. et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434, 644–648 (2005).
Little, C. B. et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 60, 3723–3733 (2009).
Goldring, M. B. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best. Pr. Res. Clin. Rheumatol. 20, 1003–1025 (2006).
Feng, X. H., Lin, X. & Derynck, R. Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15(Ink4B) transcription in response to TGF-beta. EMBO J. 19, 5178–5193 (2000).
Kavurma, M. M. & Khachigian, L. M. Sp1 inhibits proliferation and induces apoptosis in vascular smooth muscle cells by repressing p21WAF1/Cip1 transcription and cyclin D1-Cdk4-p21WAF1/Cip1 complex formation. J. Biol. Chem. 278, 32537–32543 (2003).
Zhang, L. Q. et al. Integrin-beta 1 regulates chondrocyte proliferation and apoptosis through the upregulation of GIT1 expression. Int. J. Mol. Med. 35, 1074–1080 (2015).
Chen, P., Gu, W. L., Gong, M. Z., Wang, J. & Li, D. Q. GIT1 gene deletion delays chondrocyte differentiation and healing of tibial plateau fracture through suppressing proliferation and apoptosis of chondrocyte. Bmc Musculoskel. Dis. 18, 320 (2017).
Zheng, Q. et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 7, 11215 (2016).
Wang, Y. et al. Exosomal circHIPK3 released from hypoxia-pretreated cardiomyocytes regulates oxidative damage in cardiac microvascular endothelial cells via the miR-29a/IGF-1 pathway. Oxid. Med. Cell Longev. 2019, 7954657 (2019).
Hwang, H. S. & Kim, H. A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 16, 26035–26054 (2015).
Li, X. et al. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol. Int. 45, 2096–2106 (2021).
Wang, X. et al. Exosomes isolated from bone marrow mesenchymal stem cells exert a protective effect on osteoarthritis via lncRNA LYRM4-AS1-GRPR-miR-6515-5p. Front. Cell Dev. Biol. 9, 644380 (2021).
Zhu, C., Shen, K., Zhou, W., Wu, H. & Lu, Y. Exosome-mediated circ_0001846 participates in IL-1beta-induced chondrocyte cell damage by miR-149-5p-dependent regulation of WNT5B. Clin. Immunol. 232, 108856 (2021).
Ewers, B. J., Dvoracek-Driksna, D., Orth, M. W. & Haut, R. C. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J. Orthop. Res. 19, 779–784 (2001).
Henrotin, Y. et al. Production of active oxygen species by isolated human chondrocytes. Br. J. Rheumatol. 32, 562–567 (1993).
Henrotin, Y. E., Bruckner, P. & Pujol, J. P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 11, 747–755 (2003).
Beecher, B. R., Martin, J. A., Pedersen, D. R., Heiner, A. D. & Buckwalter, J. A. Antioxidants block cyclic loading induced chondrocyte death. Iowa Orthop. J. 27, 1–8 (2007).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
Carames, B., Taniguchi, N., Otsuki, S., Blanco, F. J. & Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 62, 791–801 (2010).
Sasaki, H. et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 64, 1920–1928 (2012).
Philp, A. M., Davis, E. T. & Jones, S. W. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatol. 56, 869–881 (2017).
Millerand, M., Berenbaum, F. & Jacques, C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 37, 48–56 (2019).
Xie, J., Huang, Z., Yu, X., Zhou, L. & Pei, F. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. Cytokine Growth Factor Rev. 46, 36–44 (2019).
Asghar, S. et al. The synovial secretome contributes to cartilage pathology in osteoarthritis: a role for exosomes. Osteoarthr. Cartil. 26, S131–S132 (2018).
Kato, T. et al. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 16, R163 (2014).
Marques-Rocha, J. L. et al. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 29, 3595–3611 (2015).
Zhou, Y. et al. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Disco. 7, 37 (2021).
Tao, Y. et al. Downregulation of miR-106b attenuates inflammatory responses and joint damage in collagen-induced arthritis. Rheumatol. 56, 1804–1813 (2017).
Li, X., Zhou, Q., Tao, L. & Yu, C. MicroRNA-106a promotes cell migration and invasion by targeting tissue inhibitor of matrix metalloproteinase 2 in cervical cancer. Oncol. Rep. 38, 1774–1782 (2017).
Nakasa, T. et al. Exosome derived from osteoarthritis cartilage induces catabolic factor gene expressions in synovium. In ORS 2012 Annual Meeting, San Francisco, Poster No. 0708 (2012).
Xie, J. W. et al. Alpha defensin-1 attenuates surgically induced osteoarthritis in association with promoting M1 to M2 macrophage polarization. Osteoarthr. Cartil. 29, 1048–1059 (2021).
Sun, Y., Zuo, Z. & Kuang, Y. An emerging target in the battle against osteoarthritis: macrophage polarization. Int. J. Mol. Sci. 21, 8513 (2020).
Taylor, P. R. et al. Macrophage receptors and immune recognition. Annu Rev. Immunol. 23, 901–944 (2005).
Miao, X., Leng, X. & Zhang, Q. The current state of nanoparticle-induced macrophage polarization and reprogramming research. Int. J. Mol. Sci. 18, 336 (2017).
Zhang, H., Cai, D. & Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 28, 555–561 (2020).
Topoluk, N. et al. Amniotic mesenchymal stem cells mitigate osteoarthritis progression in a synovial macrophage-mediated in vitro explant coculture model. J. Tissue Eng. Regen. Med. 12, 1097–1110 (2018).
Wang, R. & Xu, B. TGF-beta 1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 384, 113–127 (2021).
Domenis, R. et al. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediators Inflamm. 2017, 4814987 (2017).
Bai, J. Y. et al. LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 11, 763 (2020).
Goldring, S. R. & Goldring, M. B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 12, 632–644 (2016).
Chen, L. Z. et al. Horizontal fissuring at the osteochondral interface: a novel and unique pathological feature in patients with obesity-related osteoarthritis. Ann. Rheum. Dis. 79, 811–818 (2020).
Yuan, X. L. et al. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 22, 1077–1089 (2014).
Burr, D. B. & Gallant, M. A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 8, 665–673 (2012).
Sun, W. et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Disco. 2, 16015 (2016).
Sanchez, C. et al. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 13, 988–997 (2005).
Ni, Z. et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 8, 25 (2020).
Li, Z., Huang, Z. & Bai, L. Cell interplay in osteoarthritis. Front. Cell Dev. Biol. 9, 720477 (2021).
Wu, X., Crawford, R., Xiao, Y., Mao, X. & Prasadam, I. Osteoarthritic subchondral bone release exosomes that promote cartilage degeneration. Cells 10, 251 (2021).
Liu, J. et al. Exosomal transfer of osteoclast-derived miRNAs to chondrocytes contributes to osteoarthritis progression. Nat. Aging 1, 368–384 (2021).
Simpson, R. J., Lim, J. W., Moritz, R. L. & Mathivanan, S. Exosomes: proteomic insights and diagnostic potential. Expert Rev. Proteom. 6, 267–283 (2009).
Skriner, K., Adolph, K., Jungblut, P. R. & Burmester, G. R. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 54, 3809–14 (2006).
Zheng, L. et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine 14, 3193–3212 (2019).
Gao, K. et al. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod. Rheumatol. 30, 758–764 (2020).
Zhang, S. P. et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156, 16–27 (2018).
Leung, K. et al. Prediction of total knee replacement and diagnosis of osteoarthritis by using deep learning on knee radiographs: data from the osteoarthritis initiative. Radiology 296, 584–593 (2020).
Kijowski, R., Demehri, S., Roemer, F. & Guermazi, A. Osteoarthritis year in review 2019: imaging. Osteoarthr. Cartil. 28, 285–295 (2020).
Hao, H. Q., Zhang, J. F., He, Q. Q. & Wang, Z. Cartilage oligomeric matrix protein, C-terminal cross-linking telopeptide of type II collagen, and matrix metalloproteinase-3 as biomarkers for knee and hip osteoarthritis (OA) diagnosis: a systematic review and meta-analysis. Osteoarthr. Cartil. 27, 726–736 (2019).
Huang, Z. Y. et al. Biomarkers of inflammation - LBP and TLR- predict progression of knee osteoarthritis in the DOXY clinical trial. Osteoarthr. Cartil. 26, 1658–1665 (2018).
Mobasheri, A., Bay-Jensen, A. C., van Spil, W. E., Larkin, J. & Levesque, M. C. Osteoarthritis Year in Review 2016: biomarkers (biochemical markers). Osteoarthr. Cartil. 25, 199–208 (2017).
Liu, C. G. et al. MicroRNA-135a in ABCA1-labeled exosome is a serum biomarker candidate for Alzheimer’s disease. Biomed. Environ. Sci. 34, 19–28 (2021).
Soares Martins, T. et al. Diagnostic and therapeutic potential of exosomes in Alzheimer’s disease. J. Neurochem. 156, 162–181 (2021).
Hamlett, E. D. et al. Exosomal biomarkers in Down syndrome and Alzheimer’s disease. Free Radic. Biol. Med. 114, 110–121 (2018).
Zamani, P., Fereydouni, N., Butler, A. E., Navashenaq, J. G. & Sahebkar, A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc. Med. 29, 313–323 (2019).
Zhang, Y., Hu, Y. W., Zheng, L. & Wang, Q. Characteristics and roles of exosomes in cardiovascular disease. DNA Cell Biol. 36, 202–211 (2017).
Barile, L. & Vassalli, G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharm. Ther. 174, 63–78 (2017).
Yang, D. et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics 10, 3684–3707 (2020).
Jiang, C. et al. MicroRNA-26a negatively regulates toll-like receptor 3 expression of rat macrophages and ameliorates pristane induced arthritis in rats. Arthritis Res. Ther. 16, R9 (2014).
Kolhe, R. et al. Sex-specific differences in extracellular vesicle protein cargo in synovial fluid of patients with osteoarthritis. Life 10, 337 (2020).
Zhao, Y. & Xu, J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 42, 2865–2872 (2018).
Ali, S. A. et al. Sequencing identifies a distinct signature of circulating microRNAs in early radiographic knee osteoarthritis. Osteoarthr. Cartil. 28, 1471–1481 (2020).
Beyer, C. et al. Signature of circulating microRNAs in osteoarthritis. Ann. Rheum. Dis. 74, e18 (2015).
Chen, X. et al. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 22, 256 (2020).
Jin, Z., Ren, J. A. & Qi, S. L. Exosomal miR-9-5p secreted by bone marrow-derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. 381, 99–114 (2020).
Miyaki, S. & Asahara, H. Macro view of microRNA function in osteoarthritis. Nat. Rev. Rheumatol. 8, 543–552 (2012).
Lin, T. et al. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR‑140‑5p‑overexpressing human dental pulp stem cells. Int J. Mol. Med. 47, 7 (2021).
Liang, Y. J. et al. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. Acs Appl Mater. Interfaces 12, 36938–36947 (2020).
Liu, X. et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 9, 4430–4438 (2017).
Chen, P. et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 9, 2439–2459 (2019).
Liu, Y. et al. Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am. J. Sports Med. 50, 1088–1105 (2022).
Sun, H. et al. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J. Cell Biochem. 120, 171–181 (2019).
Jin, Z., Ren, J. & Qi, S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharmacol. 78, 105946 (2020).
Song, J., Kang, Y., Chun, C.-H. & Jin, E.-J. Selective loading of exosomal HULC and miR-372 is responsible for chondrocyte death during OA pathogenesis. Anim. Cells Syst. 21, 397–403 (2017).
Dong, J., Li, L., Fang, X. & Zang, M. Exosome-encapsulated microRNA-127-3p released from bone marrow-derived mesenchymal stem cells alleviates osteoarthritis through regulating CDH11-mediated Wnt/beta-catenin pathway. J. Pain. Res. 14, 297–310 (2021).
Huang, Y. et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 promotes osteoblast proliferation and differentiation in osteoarthritis by reducing Elf3. J. Cell Mol. Med. 25, 7734–7745 (2021).
Kim, M., Shin, D. I., Choi, B. H. & Min, B. H. Exosomes from IL-1beta-primed mesenchymal stem cells inhibited IL-1beta- and TNF-alpha-mediated inflammatory responses in osteoarthritic SW982 cells. Tissue Eng. Regen. Med. 18, 525–536 (2021).
Qiu, M., Liu, D. & Fu, Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1 beta induced osteoarthritis via targeting HMGB1. Life Sci. 269, 118987 (2021).
Tao, Y. et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-kappaB signaling pathway. Bioorg. Chem. 113, 104978 (2021).
Meng, F. et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics 8, 2862–2883 (2018).
Liu, Y. et al. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 475, 3629–3638 (2018).
Yang, Q. et al. LncRNA H19 secreted by umbilical cord blood mesenchymal stem cells through microRNA-29a-3p/FOS axis for central sensitization of pain in advanced osteoarthritis. Am. J. Transl. Res. 13, 1245–1256 (2021).
Mao, G. et al. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res. Ther. 12, 389 (2021).
Tao, S. C. et al. Small extracellular vesicles in combination with sleep-related circRNA3503: A targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 6, 4455–4469 (2021).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 81974347), the Science and Technology Program of Sichuan Province (No. 2021YJ0444), China Postdoctoral Science Foundation (No.2021M702351), Post-Doctor Research Project, West China Hospital, Sichuan University (No.2020HXBH081). The authors would like to thank Jinhan Zhou from Core Facilities of West China Hospital, Sichuan University for her assistance in this study.
Author information
Authors and Affiliations
Contributions
Y.W. and J.L. wrote the manuscript. Y.Z., W.P, X.M., and K.S. edited the manuscript and designed the figures. Y.P. and B.S. contributed to the manuscript design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Y., Li, J., Zeng, Y. et al. Exosomes rewire the cartilage microenvironment in osteoarthritis: from intercellular communication to therapeutic strategies. Int J Oral Sci 14, 40 (2022). https://doi.org/10.1038/s41368-022-00187-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41368-022-00187-z
This article is cited by
-
Insights into optimizing exosome therapies for acute skin wound healing and other tissue repair
Frontiers of Medicine (2024)
-
Mesenchymal stem cells, as glioma exosomal immunosuppressive signal multipliers, enhance MDSCs immunosuppressive activity through the miR-21/SP1/DNMT1 positive feedback loop
Journal of Nanobiotechnology (2023)
-
A pH-responsive metal-organic framework for the co-delivery of HIF-2α siRNA and curcumin for enhanced therapy of osteoarthritis
Journal of Nanobiotechnology (2023)