Abstract
During the past decade, research has revealed that the vast community of micro-organisms that inhabit the gut — known as the gut microbiota — is intricately linked to human health and disease, partly as a result of its influence on systemic immune responses. Accumulating evidence demonstrates that these effects on immune function are important in neuroinflammatory diseases, such as multiple sclerosis (MS), and that modulation of the microbiome could be therapeutically beneficial in these conditions. In this Review, we examine the influence that the gut microbiota have on immune function via modulation of serotonin production in the gut and through complex interactions with components of the immune system, such as T cells and B cells. We then present evidence from studies in mice and humans that these effects of the gut microbiota on the immune system are important in the development and course of MS. We also consider how strategies for manipulating the composition of the gut microbiota could be used to influence disease-related immune dysfunction and form the basis of a new class of therapeutics. The strategies discussed include the use of probiotics, supplementation with bacterial metabolites, transplantation of faecal matter or defined microbial communities, and dietary intervention. Carefully designed studies with large human cohorts will be required to gain a full understanding of the microbiome changes involved in MS and to develop therapeutic strategies that target these changes.
Key points
-
The colony of bacteria that inhabit the gut — known as the gut microbiota — varies in composition according to genetic factors and, more importantly, envorinmental influences, particularly diet.
-
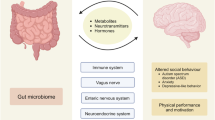
The composition of the gut microbiota influences the production of serotonin in the gut, which in turn influences serotonin-mediated regulation of systemic immune function.
-
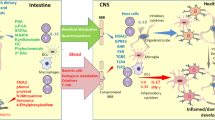
Gut microbiota are also involved in complex interactions with the gut and immune cells in the small intestine and the colon, thereby influencing immune responses in the periphery and the central nervous system.
-
Abundant evidence indicates that the gut microbiota has a role in multiple sclerosis (MS) through its influence on immune function.
-
Therapeutic strategies that target the microbiota — including dietary interventions, probiotics, short-chain fatty acids and faecal microbial transplantation — seem promising for the treatment of MS, but further work is needed to assess their effectiveness.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cryan, J. F. et al. The microbiota–gut–brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Clemente, J. C., Ursell, L. K., Parfrey, L. W. & Knight, R. The impact of the gut microbiota on human health: an integrative view. Cell 148, 1258–1270 (2012).
Ochoa-Reparaz, J. et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183, 6041–6050 (2009).
Mahr, B. & Wekerle, T. Murine models of transplantation tolerance through mixed chimerism: advances and roadblocks. Clin. Exp. Immunol. 189, 181–189 (2017).
Honda, K. & Littman, D. R. The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016).
Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32 (2015).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Cekanaviciute, E. et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1711235114 (2017).
Lee, Y. K. & Mazmanian, S. K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 330, 1768–1773 (2010).
Ochoa-Reparaz, J. et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3, 487–495 (2010).
Mawe, G. M. & Hoffman, J. M. Serotonin signalling in the gut — functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486 (2013).
Wan, M., Ding, L., Wang, D., Han, J. & Gao, P. Serotonin: a potent immune cell modulator in autoimmune diseases. Front. Immunol. 11, 186 (2020).
Mossner, R. & Lesch, K. P. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav. Immun. 12, 249–271 (1998).
Fiebich, B. L. et al. Expression of 5-HT3A receptors in cells of the immune system. Scand. J. Rheumatol. Suppl. 119, 9–11 (2004).
Jang, S. W. et al. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl Acad. Sci. USA 107, 3876–3881 (2010).
Farez, M. F. et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162, 1338–1352 (2015).
Gershon, M. D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 20, 14–21 (2013).
Stone, T. W. & Darlington, L. G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 1, 609–620 (2002).
Cote, F. et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl Acad. Sci. USA 100, 13525–13530 (2003).
Clarke, G. et al. The microbiome–gut–brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiat. 18, 666–673 (2013).
Wikoff, W. R. et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl Acad. Sci. USA 106, 3698–3703 (2009).
Reigstad, C. S. et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403 (2015).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Raboni, S., Bettati, S. & Mozzarelli, A. Tryptophan synthase: a mine for enzymologists. Cell Mol. Life Sci. 66, 2391–2403 (2009).
Israelyan, N. & Margolis, K. G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 132, 1–6 (2018).
Bartolomucci, A. et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE https://doi.org/10.1371/journal.pone.0003301 (2008).
Yaghoubfar, R. et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut–brain axis in mice. Sci. Rep. https://doi.org/10.1038/s41598-020-79171-8 (2020).
Wen, J. et al. Efficacy of N-acetylserotonin and melatonin in the EAE model of multiple sclerosis. J. NeuroImmune Pharmacol. 11, 763–773 (2016).
Sacramento, P. M. et al. Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4+ T-cell subsets in multiple sclerosis patients. Eur. J. Immunol. 48, 1376–1388 (2018).
de las Casas-Engel, M. et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J. Immunol. 190, 2301–2310 (2013).
Zeinstra, E. M. et al. 5HT4 agonists inhibit interferon-γ-induced MHC class II and B7 costimulatory molecules expression on cultured astrocytes. J. Neuroimmunol. 179, 191–195 (2006).
Jang, S. W. et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl Acad. Sci. USA 107, 2687–2692 (2010).
Aminian, A. et al. Tropisetron diminishes demyelination and disease severity in an animal model of multiple sclerosis. Neuroscience 248, 299–306 (2013).
Nakatani, Y. et al. Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat. Eur. J. Neurosci. 27, 2466–2472 (2008).
Desbonnet, L., Garrett, L., Clarke, G., Bienenstock, J. & Dinan, T. G. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J. Psychiat. Res. 43, 164–174 (2008).
Rothhammer, V. & Quintana, F. J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 19, 184–197 (2019).
Geuking, M. B., McCoy, K. D. & Macpherson, A. J. The continuum of intestinal CD4+ T cell adaptations in host–microbial mutualism. Gut Microbes 2, 353–357 (2011).
Zelante, T. et al. Impaired calcineurin signaling in myeloid cells results in downregulation of pentraxin-3 and increased susceptibility to aspergillosis. Mucosal Immunol. 10, 470–480 (2017).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Yang, Y. et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 510, 152–156 (2014).
Lee, Y. K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108, 4615–4622 (2011).
Cosorich, I. et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 3, e1700492 (2017).
Tan, T. G. et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc. Natl Acad. Sci. USA 113, E8141–E8150 (2016).
Magarian Blander, J., Longman, R. S., Iliev, I. D., Sonnenberg, G. F. & Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 18, 851–860 (2017).
Jangi, S. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016).
Geuking, M. B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011).
Park, J. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8, 80–93 (2015).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Round, J. L. et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Jeon, S. G. et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 8, e1002714 (2012).
Cebula, A. et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 497, 258–262 (2013).
Sefik, E. et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349, 993–997 (2015).
Josefowicz, S. Z. et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 (2012).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Wang, H. C. et al. Anti-proliferation effect on human breast cancer cells via inhibition of pRb phosphorylation by taiwanin E isolated from Eleutherococcus trifoliatus. Nat. Prod. Commun. 9, 1303–1306 (2014).
Takenaka, M. C., Robson, S. & Quintana, F. J. Regulation of the T cell response by CD39. Trends Immunol. 37, 427–439 (2016).
Ochoa-Reparaz, J. et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 185, 4101–4108 (2010).
Wang, J. et al. Dietary history contributes to enterotype-like clustering and functional metagenomic content in the intestinal microbiome of wild mice. Proc. Natl Acad. Sci. USA 111, E2703–E2710 (2014).
Wang, H. et al. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS ONE 9, e90153 (2014).
Fletcher, J. M. et al. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J. Immunol. 183, 7602–7610 (2009).
Carnero Contentti, E., Farez, M. F. & Correale, J. Mucosal-associated invariant T cell features and TCR repertoire characteristics during the course of multiple sclerosis. Front. Immunol. 10, 2690 (2019).
Napier, R. J., Adams, E. J., Gold, M. C. & Lewinsohn, D. M. The role of mucosal associated invariant T cells in antimicrobial immunity. Front. Immunol. 6, 344 (2015).
Ben Youssef, G. et al. Ontogeny of human mucosal-associated invariant T cells and related T cell subsets. J. Exp. Med. 215, 459–479 (2018).
Tastan, C. et al. Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol. 11, 1591–1605 (2018).
Dias, J., Leeansyah, E. & Sandberg, J. K. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl Acad. Sci. USA 114, E5434–E5443 (2017).
Held, K., Beltran, E., Moser, M., Hohlfeld, R. & Dornmair, K. T-cell receptor repertoire of human peripheral CD161hiTRAV1-2+MAIT cells revealed by next generation sequencing and single cell analysis. Hum. Immunol. 76, 607–614 (2015).
Mei, H. E. et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood 113, 2461–2469 (2009).
Mantis, N. J., Rol, N. & Corthesy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611 (2011).
Mortha, A. et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288 (2014).
Zeng, B. et al. ILC3 function as a double-edged sword in inflammatory bowel diseases. Cell Death Dis. 10, 315 (2019).
Rojas, O. L. et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 176, 610–624.e618 (2019).
Probstel, A. K. et al. Gut microbiota-specific IgA+ B cells traffic to the CNS in active multiple sclerosis. Sci. Immunol. https://doi.org/10.1126/sciimmunol.abc7191 (2020).
Fitzpatrick, Z. et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature 587, 472–476 (2020).
McPherson, A. C., Pandey, S. P., Bender, M. J. & Meisel, M. Systemic immunoregulatory consequences of gut commensal translocation. Trends Immunol. 42, 137–150 (2021).
Johansson, M. E. & Hansson, G. C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 16, 639–649 (2016).
Nouri, M., Bredberg, A., Westrom, B. & Lavasani, S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS ONE 9, e106335 (2014).
Coureuil, M., Lecuyer, H., Bourdoulous, S. & Nassif, X. A journey into the brain: insight into how bacterial pathogens cross blood–brain barriers. Nat. Rev. Microbiol. 15, 149–159 (2017).
Banerjee, A. et al. Bacterial pili exploit integrin machinery to promote immune activation and efficient blood–brain barrier penetration. Nat. Commun. 2, 462 (2011).
Boveri, M. et al. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood–brain barrier disruption through glia activation: role of pro-inflammatory cytokines and nitric oxide. Neuroscience 137, 1193–1209 (2006).
Braniste, T. et al. Viability and proliferation of endothelial cells upon exposure to GaN nanoparticles. Beilstein J. Nanotechnol. 7, 1330–1337 (2016).
Schilderink, R. et al. The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G1138–G1146 (2016).
Braniste, V. et al. The gut microbiota influences blood–brain barrier permeability in mice. Sci. Transl. Med. 6, 263ra158 (2014).
Zheng, L. et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of Claudin-2. J. Immunol. 199, 2976–2984 (2017).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Matcovitch-Natan, O. et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016).
Nayak, D., Roth, T. L. & McGavern, D. B. Microglia development and function. Annu. Rev. Immunol. 32, 367–402 (2014).
Brown, D. G. et al. The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. eLife https://doi.org/10.7554/eLife.47117 (2019).
Luck, B. et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci. Rep. 10, 7737 (2020).
Goverman, J. et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell 72, 551–560 (1993).
Yokote, H. et al. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 173, 1714–1723 (2008).
Wang, Y. & Kasper, L. H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 38, 1–12 (2014).
Wekerle, H., Flugel, A., Fugger, L., Schett, G. & Serreze, D. Autoimmunity’s next top models. Nat. Med. 18, 66–70 (2012).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Mirza, A. & Mao-Draayer, Y. The gut microbiome and microbial translocation in multiple sclerosis. Clin. Immunol. https://doi.org/10.1016/j.clim.2017.03.001 (2017).
Probstel, A. K. & Baranzini, S. E. The role of the gut microbiome in multiple sclerosis risk and progression: towards characterization of the “MS microbiome”. Neurotherapeutics 15, 126–134 (2018).
Berer, K. et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1711233114 (2017).
Liu, S. et al. Oral administration of miR-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding Akkermansia muciniphila. Cell Host Microbe 26, 779–794 e778 (2019).
Bian, X. et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front. Microbiol. 10, 2259 (2019).
Huck, O. et al. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J. Clin. Periodontol. 47, 202–212 (2020).
Zhu, L. et al. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe−/− mice. Atherosclerosis 268, 117–126 (2018).
Wu, W. et al. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front. Microbiol. 8, 1804 (2017).
Zhou, Q. et al. Gut bacteria Akkermansia is associated with reduced risk of obesity: evidence from the American gut project. Nutr. Metab. 17, 90 (2020).
Reynders, T. et al. Gut microbiome variation is associated to multiple sclerosis phenotypic subtypes. Ann. Clin. Transl. Neurol. 7, 406–419 (2020).
Cox, L. M. et al. Gut microbiome in progressive multiple sclerosis. Ann. Neurol. 89, 1195–1211 (2021).
Takewaki, D. et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc. Natl Acad. Sci. USA 117, 22402–22412 (2020).
Leeming, E. R., Johnson, A. J., Spector, T. D. & Le Roy, C. I. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients https://doi.org/10.3390/nu11122862 (2019).
Rothschild, D. et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 (2018).
Turnbaugh, P. J. et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1, 6ra14 (2009).
O’Toole, P. W. Changes in the intestinal microbiota from adulthood through to old age. Clin. Microbiol. Infect. 18, 44–46 (2012).
Fransen, F. et al. The impact of gut microbiota on gender-specific differences in immunity. Front. Immunol. https://doi.org/10.3389/fimmu.2017.00754 (2017).
Gupta, V. K., Paul, S. & Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. https://doi.org/10.3389/fmicb.2017.01162 (2017).
Dasgupta, S. et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE https://doi.org/10.1371/journal.pone.0171352 (2017).
The iMSMS Consortium. Household paired design reduces variance and increases power in multi-city gut microbiome study in multiple sclerosis. Mult. Scler. https://doi.org/10.1177/1352458520924594 (2020).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Storoni, M. & Plant, G. T. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult. Scler. Int. 2015, 681289 (2015).
Barone, M. et al. Gut microbiome response to a modern Paleolithic diet in a Western lifestyle context. PLoS ONE 14, e0220619 (2019).
Wahls, T. L. et al. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: the WAVES randomized parallel-arm clinical trial. Mult. Scler. J. Exp. Transl. Clin. 7, 20552173211035399 (2021).
Mousavi-Shirazi-Fard, Z., Mazloom, Z., Izadi, S. & Fararouei, M. The effects of modified anti-inflammatory diet on fatigue, quality of life, and inflammatory biomarkers in relapsing–remitting multiple sclerosis patients: a randomized clinical trial. Int. J. Neurosci. 131, 657–665 (2021).
Frank, J., Gupta, A., Osadchiy, V. & Mayer, E. A. Brain–gut–microbiome interactions and intermittent fasting in obesity. Nutrients https://doi.org/10.3390/nu13020584 (2021).
Hill, C. et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
Klingensmith, N. J. & Coopersmith, C. M. The gut as the motor of multiple organ dysfunction in critical illness. Crit. Care Clin. 32, 203–212 (2016).
Khailova, L., Petrie, B., Baird, C. H., Dominguez Rieg, J. A. & Wischmeyer, P. E. Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis. PLoS ONE 9, e97861 (2014).
Krezalek, M. A., DeFazio, J., Zaborina, O., Zaborin, A. & Alverdy, J. C. The shift of an intestinal “microbiome” to a “pathobiome” governs the course and outcome of sepsis following surgical injury. Shock 45, 475–482 (2016).
Lavasani, S. et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 5, e9009 (2010).
Ezendam, J. & van Loveren, H. Lactobacillus casei Shirota administered during lactation increases the duration of autoimmunity in rats and enhances lung inflammation in mice. Br. J. Nutr. 99, 83–90 (2008).
Takata, K. et al. The lactic acid bacterium Pediococcus acidilactici suppresses autoimmune encephalomyelitis by inducing IL-10-producing regulatory T cells. PLoS ONE 6, e27644 (2011).
Rezende, R. M. et al. Hsp65-producing Lactococcus lactis prevents experimental autoimmune encephalomyelitis in mice by inducing CD4+LAP+ regulatory T cells. J. Autoimmun. 40, 45–57 (2013).
Kouchaki, E. et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 36, 1245–1249 (2017).
Tankou, S. K. et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann. Neurol. 83, 1147–1161 (2018).
Suez, J., Zmora, N., Segal, E. & Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729 (2019).
Young, L. J., McFarlane, R., Slender, A. L. & Deane, E. M. Histological and immunohistological investigation of the lymphoid tissue in normal and mycobacteria-affected specimens of the Rufous Hare-wallaby (Lagorchestes hirsutus). J. Anat. 202, 315–325 (2003).
Li, H. Y. et al. Modulation of gut microbiota, short-chain fatty acid production, and inflammatory cytokine expression in the cecum of porcine deltacoronavirus-infected chicks. Front. Microbiol. 11, 897 (2020).
Haase, S., Haghikia, A., Gold, R. & Linker, R. A. Dietary fatty acids and susceptibility to multiple sclerosis. Mult. Scler. 24, 12–16 (2018).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Wang, G. et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc. Natl Acad. Sci. USA 112, 2853–2858 (2015).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015).
Furusawa, Y. et al. Commensal-microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Segain, J. P. et al. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn’s disease. Gut 47, 397–403 (2000).
Brown, A. J. et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 (2003).
Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014).
Park, J., Goergen, C. J., HogenEsch, H. & Kim, C. H. Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis. J. Immunol. 196, 2388–2400 (2016).
Hong, Y. H. et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146, 5092–5099 (2005).
Chen, S. et al. Effect of inhibiting the signal of mammalian target of rapamycin on memory T cells. Transpl. Proc. 46, 1642–1648 (2014).
Delgoffe, G. M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Jin, U. H. et al. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 85, 777–788 (2014).
Singh, N. P. et al. Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of microRNA. J. Immunol. 196, 1108–1122 (2016).
Denison, M. S., Soshilov, A. A., He, G., DeGroot, D. E. & Zhao, B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 124, 1–22 (2011).
Stevens, E. A., Mezrich, J. D. & Bradfield, C. A. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127, 299–311 (2009).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Cammarota, G. et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 41, 835–843 (2015).
Kociolek, L. K. & Gerding, D. N. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nat. Rev. Gastroenterol. Hepatol. 13, 150–160 (2016).
Singh, R., Nieuwdorp, M., ten Berge, I. J., Bemelman, F. J. & Geerlings, S. E. The potential beneficial role of faecal microbiota transplantation in diseases other than Clostridium difficile infection. Clin. Microbiol. Infect. 20, 1119–1125 (2014).
Vendrik, K. E. W. et al. Fecal microbiota transplantation in neurological disorders. Front. Cell Infect. Microbiol. 10, 98 (2020).
Stein, R. R. et al. Computer-guided design of optimal microbial consortia for immune system modulation. eLife https://doi.org/10.7554/eLife.30916 (2018).
Belbasis, L. & Bellou, V. in Genetic Epidemiology Ch. 1, 1–6 (Springer, 2018).
Sedgwick, P. Bias in observational study designs: cross sectional studies. BMJ 350, h1286–h1286 (2015).
Sibbald, B. & Roland, M. Understanding controlled trials: why are randomised controlled trials important? BMJ 316, 201–201 (1998).
Beaumont, M. et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 106, 1005–1019 (2017).
Vandeputte, D. et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 66, 1968–1974 (2017).
Moher, D. et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 63, e1–e37 (2010).
Cryan, J. F., O’Riordan, K. J., Sandhu, K., Peterson, V. & Dinan, T. G. The gut microbiome in neurological disorders. Lancet Neurol. 19, 179–194 (2020).
Cantarel, B. L. et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J. Investig. Med. 63, 729–734 (2015).
Miyake, S. et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS ONE 10, e0137429 (2015).
Chen, J. et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. https://doi.org/10.1038/srep28484 (2016).
Tremlett, H. et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur. J. Neurol. 23, 1308–1321 (2016).
Ling, Z. et al. Alterations of the fecal microbiota in Chinese patients with multiple sclerosis. Front. Immunol. 11, 590783 (2020).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks A. Mangalam and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Correale, J., Hohlfeld, R. & Baranzini, S.E. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol 18, 544–558 (2022). https://doi.org/10.1038/s41582-022-00697-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-022-00697-8
This article is cited by
-
Research Hotspots and Frontiers of Alzheimer’s Disease and Gut Microbiota: A Knowledge Mapping and Text Mining Analysis
Molecular Neurobiology (2024)
-
A metabolite from commensal Candida albicans enhances the bactericidal activity of macrophages and protects against sepsis
Cellular & Molecular Immunology (2023)
-
Modelling host–microbiome interactions in organ-on-a-chip platforms
Nature Reviews Bioengineering (2023)
-
T cell–microbiome communication influences disease in MS model
Nature Reviews Neurology (2023)
-
Autoimmune diseases and gut microbiota: a bibliometric and visual analysis from 2004 to 2022
Clinical and Experimental Medicine (2023)