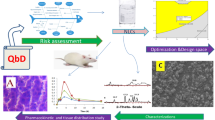
Abstract
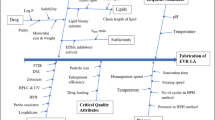
The therapeutic potential of atazanavir (BCS Class II drug), a highly selective inhibitor of human immunodeficiency virus (HIV-1), has been largely limited due to its low intrinsic solubility at elevated pH resulting in low oral bioavailability. Thus, the current work describes the systematic development, optimization, and evaluation of hydroxypropyl methylcellulose acetate succinate (HPMC-AS)-based supersaturable preconcentrate isotropic mixture (SP-IM) containing long-chain triglyceride to improve intestinal lymphatic transport and augment oral bioavailability of atazanavir (ATZ). A D-optimal mixture design was employed for optimization of plain IM containing corn oil, oleic acid, Tween 80, and propylene glycol, evaluating various critical quality attributes (CQAs) like particle size, polydispersity index, self-emulsification time, % transmittance, and drug content. In silico analysis and in vitro supersaturation test facilitated the selection of HPMC-AS as a best suited polymeric precipitation inhibitor (PPI) for formulating ATZ loaded SP-IM (ATZ-SP-IM). In vitro dissolution data indicated that ATZ-SP-IM exhibits superior performance in 0.025 N HCl and pH 6.8 over pure drug. Ex vivo permeation and in vivo pharmacokinetic study of ATZ-SP-IM corroborated enhanced permeation (2.03 fold) and improved drug absorption via lymphatic transport in Wistar rats. Further, the pharmacokinetic performance of ATZ-SP-IM was not affected in presence of H2 receptor antagonist. Therefore, the results showed that ATZ-SP-IM can significantly improve the biopharmaceutical attributes of ATZ so as to lay a foundation of further research on the new dosage form of ATZ.
Graphical abstract





Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Khan SA, Rehman S, Nabi B, Iqubal A, Nehal N, Fahmy UA, Kotta S, Baboota S, Md S, Ali J. Boosting the brain delivery of Atazanavir through nanostructured lipid carrier-based approach for mitigating neuroaids. Pharmaceutics. 2020;12:1059.
Sarma A, Das MK. Formulation by Design (FbD) approach to develop Tenofovir Disoproxil Fumarate loaded nanostructured lipid carriers (NLCs) for the aptness of nose to brain delivery. J Drug Deliv Ther. 2019;9:148–59.
Bagasra O. A unified concept of HIV latency. Expert Opin Biol Ther. 2006;6:1135–49.
Ojewole E, Mackraj I, Naidoo P, Govender T. Exploring the use of novel drug delivery systems for antiretroviral drugs. Eur J Pharm Biopharm. 2008;70:697–710.
Naidoo P. Barriers to HIV care and treatment by doctors: a review of the literature. S Afr Fam Pract. 2006;48:1.
Lei B, Zha W, Wang Y, Wen C, Studer EJ, Wang X, Jin F, Wang G, Zhang L, Zhou H. Development of a novel self-microemulsifying drug delivery system for reducing HIV protease inhibitor-induced intestinal epithelial barrier dysfunction. Mol Pharm. 2010;7:844–53.
Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400.
Fukushima K, Terasaka S, Haraya K, Kodera S, Seki Y, Wada A, Ito Y, Shibata N, Sugioka N, Takada K. Pharmaceutical approach to HIV protease inhibitor atazanavir for bioavailability enhancement based on solid dispersion system. Biol Pharm Bull. 2007;30:733–8.
Colonno R, Rose R, McLaren C, Thiry A, Parkin N, Friborg J. Identification of I50L as the signature atazanavir (ATV)-resistance mutation in treatment-naive HIV-1-infected patients receiving ATV-containing regimens. J Infect Dis. 2004;189:1802–10.
Wood R, Phanuphak P, Cahn P, Pokrovskiy V, Rozenbaum W, Pantaleo G, Sension M, Murphy R, Mancini M, Kelleher T. Long-term efficacy and safety of atazanavir with stavudine and lamivudine in patients previously treated with nelfinavir or atazanavir. JAIDS J Acquir Immune Defic Syndr. 2004;36:684–92.
Singh G, Pai RS. Optimized self-nanoemulsifying drug delivery system of atazanavir with enhanced oral bioavailability: in vitro/in vivo characterization. Expert Opin Drug Deliv. 2014;11:1023–32.
Saxena A, Shah D, Padmanabhan S, Gautam SS, Chowan GS, Mandlekar S, Desikan S. Prediction of pH dependent absorption using in vitro, in silico, and in vivo rat models: Early liability assessment during lead optimization. Eur J Pharm Sci. 2015;76:173–80.
Lahner E, Annibale B, Delle Fave G. Systematic review: impaired drug absorption related to the co‐administration of antisecretory therapy. Aliment Pharmacol Ther. 2009;29:1219–1229.
Tomilo DL, Smith PF, Ogundele AB, Difrancesco R, Berenson CS, Eberhardt E, Bednarczyk E, Morse GD. Inhibition of atazanavir oral absorption by lansoprazole gastric acid suppression in healthy volunteers. Pharmacotherapy: J Hum Pharmacol Drug Ther. 2006;26:341–346.
Xia X, Zhou C, Ballell L, Garcia-Bennett AE. In vivo enhancement in bioavailability of atazanavir in the presence of proton-pump inhibitors using mesoporous materials. Chem Med Chem. 2012;7:43–8.
DeVault KR, Talley NJ. Insights into the future of gastric acid suppression. Nat Rev Gastroenterol Hepatol. 2009;6:524–32.
Khanlou H, Farthing C. Co-administration of atazanavir with proton-pump inhibitors and H2 blockers. JAIDS J Acquir Immune Defic Syndr. 2005;39:503.
Chattopadhyay N, Zastre J, Wong H-L, Wu XY, Bendayan R. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm Res. 2008;25:2262–71.
Morgen M, Saxena A, Chen X-Q, Miller W, Nkansah R, Goodwin A, Cape J, Haskell R, Su C, Gudmundsson O. Lipophilic salts of poorly soluble compounds to enable high-dose lipidic SEDDS formulations in drug discovery. Eur J Pharm Biopharm. 2017;117:212–23.
Singh G, Pai RS. Atazanavir-loaded Eudragit RL 100 nanoparticles to improve oral bioavailability: optimization and in vitro/in vivo appraisal. Drug Delivery. 2016;23:532–9.
Holm R, Porter CJ, Edwards GA, Müllertz A, Kristensen HG, Charman WN. Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides. Eur J Pharm Sci. 2003;20:91–7.
Zhang N, Zhang W, Jin Y, Quan D-Q. Studies on preparation of carbamazepine (CBZ) supersaturatable self-microemulsifying (S-SMEDDS) formulation and relative bioavailability in beagle dogs. Pharm Dev Technol. 2011;16:415–21.
Jain A, Kaur R, Beg S, Kushwah V, Jain S, Singh B. Novel cationic supersaturable nanomicellar systems of raloxifene hydrochloride with enhanced biopharmaceutical attributes. Drug Deliv Transl Res. 2018;8:670–92.
Jo K, Kim H, Khadka P, Jang T, Kim SJ, Hwang S-H, Lee J. Enhanced intestinal lymphatic absorption of saquinavir through supersaturated self-microemulsifying drug delivery systems. Asian J Pharm Sci. 2020;15:336–46.
Krishna KV, Saha RN, Singhvi G, Dubey SK. Pre-clinical pharmacokinetic-pharmacodynamic modelling and biodistribution studies of donepezil hydrochloride by a validated HPLC method. RSC Adv. 2018;8:24740–9.
Kamboj S, Sethi S, Rana V. Lipid based delivery of Efavirenz: an answer to its erratic absorption and food effect. Eur J Pharm Sci. 2018;123:199–216.
Gao H, Jia H, Dong J, Yang X, Li H, Ouyang D. Integrated in silico formulation design of self-emulsifying drug delivery systems. Acta Pharmaceutica Sinica B. 2021;11:3585–94.
Kamboj S, Rana V. Quality-by-design based development of a self-microemulsifying drug delivery system to reduce the effect of food on Nelfinavir mesylate. Int J Pharm. 2016;501:311–25.
Kamble RN, Mehta PP, Kumar A. Efavirenz self-nano-emulsifying drug delivery system: in vitro and in vivo evaluation. AAPS Pharm Sci Tech. 2016;17:1240–7.
Baheti A, Srivastava S, Sahoo D, Lowalekar R, Prasad Panda B, Kumar Padhi B, Raghuvanshi R. Development and pharmacokinetic evaluation of industrially viable self-microemulsifying drug delivery systems (SMEDDS) for terbinafine. Curr Drug Deliv. 2016;13:65–75.
Khurana RK, Bansal AK, Beg S, Burrow AJ, Katare O, Singh KK, Singh B. Enhancing biopharmaceutical attributes of phospholipid complex-loaded nanostructured lipidic carriers of mangiferin: systematic development, characterization and evaluation. Int J Pharm. 2017;518:289–306.
Li C, Wang J-X, Le Y, Chen J-F. Studies of bicalutamide–excipients interaction by combination of molecular docking and molecular dynamics simulation. Mole Pharm. 2013;10:2362–2369.
Geetha P, Sivaram AJ, Jayakumar R, Mohan CG. Integration of in silico modeling, prediction by binding energy and experimental approach to study the amorphous chitin nanocarriers for cancer drug delivery. Carbohyd Polym. 2016;142:240–9.
Patel MR, Lamprou DA, Vavia PR. Synthesis, characterization, and drug delivery application of self-assembling amphiphilic cyclodextrin. AAPS Pharm Sci Tech. 2020;21:1–16.
Choudhary S, Silakari O. hCES1 and hCES2 mediated activation of epalrestat-antioxidant mutual prodrugs: Unwinding the hydrolytic mechanism using in silico approaches. J Mol Graph Model. 2019;91:148–63.
Dhumal DM, Akamanchi K. Self-microemulsifying drug delivery system for camptothecin using new bicephalous heterolipid with tertiary-amine as branching element. Int J Pharm. 2018;541:48–55.
Sun DD, Wen H, Taylor LS. Non-sink dissolution conditions for predicting product quality and in vivo performance of supersaturating drug delivery systems. J Pharm Sci. 2016;105:2477–88.
Hate SS, Reutzel-Edens SM, Taylor LS. Interplay of adsorption, supersaturation and the presence of an absorptive sink on drug release from mesoporous silica-based formulations. Pharm Res. 2020;37:1–18.
Bandyopadhyay S, Katare O, Singh B. Development of optimized supersaturable self-nanoemulsifying systems of ezetimibe: effect of polymers and efflux transporters. Expert Opin Drug Deliv. 2014;11:479–92.
Jaisamut P, Wiwattanawongsa K, Graidist P, Sangsen Y, Wiwattanapatapee R. Enhanced oral bioavailability of curcumin using a supersaturatable self-microemulsifying system incorporating a hydrophilic polymer; in vitro and in vivo investigations. AAPS Pharm Sci Tech. 2018;19:730–40.
Kamboj S, Sharma R, Singh K, Rana V. Aprepitant loaded solid preconcentrated microemulsion for enhanced bioavailability: a comparison with micronized aprepitant. Eur J Pharm Sci. 2015;78:90–102.
Ghai D, Sinha VR. Nanoemulsions as self-emulsified drug delivery carriers for enhanced permeability of the poorly water-soluble selective β1-adrenoreceptor blocker Talinolol. Nanomed: Nanotechnol Biol Med. 2012;8:618–26.
Patel MH, Mundada VP, Sawant KK. Novel drug delivery approach via self-microemulsifying drug delivery system for enhancing oral bioavailability of asenapine maleate: optimization, characterization, cell uptake, and in vivo pharmacokinetic studies. AAPS Pharm Sci Tech. 2019;20:1–8.
Yadav M, Trivedi V, Upadhyay V, Shah G, Baxi GA, Goswami S, Shrivastav PS. Comparison of extraction procedures for assessment of matrix effect for selective and reliable determination of atazanavir in human plasma by LC–ESI-MS/MS. J Chromatogr B. 2012;885:138–49.
Lawless E, Griffin BT, O’Mahony A, O’Driscoll CM. Exploring the impact of drug properties on the extent of intestinal lymphatic transport-in vitro and in vivo studies. Pharm Res. 2015;32:1817–29.
Constantinides PP, Wasan KM. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: in vitro/in vivo case studies. J Pharm Sci. 2007;96:235–48.
Li C-X, Wang H-B, Oppong D, Wang J-X, Chen J-F, Le Y. Excipient-assisted vinpocetine nanoparticles: experiments and molecular dynamic simulations. Mol Pharm. 2014;11:4023–35.
Zhang S, Sun M, Zhao Y, Song X, He Z, Wang J, Sun J. Molecular mechanism of polymer-assisting supersaturation of poorly water-soluble loratadine based on experimental observations and molecular dynamic simulations. Drug Deliv Transl Res. 2017;7:738–49.
Sun M, Wu C, Fu Q, Di D, Kuang X, Wang C, He Z, Wang J, Sun J. Solvent-shift strategy to identify suitable polymers to inhibit humidity-induced solid-state crystallization of lacidipine amorphous solid dispersions. Int J Pharm. 2016;503:238–46.
Parmar N, Singla N, Amin S, Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf, B. 2011;86:327–38.
Kakumanu S, Tagne JB, Wilson TA, Nicolosi RJ. A nanoemulsion formulation of dacarbazine reduces tumor size in a xenograft mouse epidermoid carcinoma model compared to dacarbazine suspension. Nanomed: Nanotechnol Biol Med. 2011;7:277–83.
Omari-Siaw E, Zhu Y, Wang H, Peng W, Firempong CK, Wang YW, Cao X, Deng W, Yu J, Xu X. Hypolipidemic potential of perillaldehyde-loaded self-nanoemulsifying delivery system in high-fat diet induced hyperlipidemic mice: formulation, in vitro and in vivo evaluation. Eur J Pharm Sci. 2016;85:112–22.
Li F, Song S, Guo Y, Zhao Q, Zhang X, Pan W, Yang X. Preparation and pharmacokinetics evaluation of oral self-emulsifying system for poorly water-soluble drug Lornoxicam. Drug Deliv. 2015;22:487–98.
Wooster TJ, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24:12758–65.
Goo YT, Song SH, Yeom DW, Chae BR, Yoon HY, Kim CH, Park SY, Kang TH, Lee S, Choi YW. Enhanced oral bioavailability of valsartan in rats using a supersaturable self-microemulsifying drug delivery system with P-glycoprotein inhibitors. Pharm Dev Technol. 2020;25:178–86.
Acknowledgements
The authors would like to acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing Direct-SRF, sanction no 09/140(0176)/2019-EMR-I. The generous supply of atazanavir sulfate from Sun Pharmaceutical Industries Ltd., Gurugram, India, is also appreciated. The authors are also thankful to Gattefosse, BASF, and Croda Chemicals, India, for their kind donation of the surfactants and the oil used in this study.
Funding
This research work was supported by Council of Scientific and Industrial Research (CSIR), New Delhi, India (Grant No. 09/140(0176)/2019-EMR-I).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This article was preprinted on Research Square preprint server (https://doi.org/10.21203/rs.3.rs-1149372/v1).
Ethical approval
The research work involving ex vivo permeation and in vivo pharmacokinetics adheres to the guidelines for care and use of the laboratory animals. Thus, all the animal investigations were performed as per the requisite protocol approved by the Institutional Animal Ethics Committee (IAEC), Punjabi University, Patiala, India (Approval No. 107/99/CPCSEA/2018–05).
Consent for publication
No human volunteers or data related to human volunteers were involved in this research work, as the proposed study was performed on rodents. Therefore, no consent was taken from human volunteers to publish manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sethi, S., Rana, V. In silico–assisted development of supersaturable preconcentrated isotropic mixture of atazanavir for augmenting biopharmaceutical performance in the presence of H2-receptor antagonist. Drug Deliv. and Transl. Res. 13, 339–355 (2023). https://doi.org/10.1007/s13346-022-01210-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01210-w