Abstract
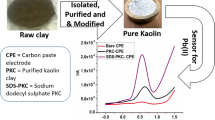
Methoxykaolinite is a very popular organo-modified kaolinite. Even though it has a number of interesting properties, this nanohybrid material is still underused in terms of practical applications. In the present study, methoxykaolinite was synthesized and used for the first time as an electrode modifier for Pb(II) determination in various aqueous media. X-ray powder diffractometry (XRD), 13C nuclear magnetic resonance (NMR), and Fourier-transform infrared (FTIR) spectroscopy were used as characterization tools to confirm the presence of grafted methoxy groups in the interlayer space of kaolinite. The electrochemical characterization of methoxykaolinite using the cationic probe Ru(NH3)63+ showed that the modified clay presents favorable interactions with cationic compounds. A methoxykaolinite-modified electrode was applied successfully to the quantification of Pb(II) in aqueous solution. At optimized experimental conditions, the calibration curve in the concentration range 0.025–0.3 μM showed excellent linearity (R2 > 0.99), a sensitivity of 3.36 μA μM–1, and a detection limit of 5.6 nM. This detection limit was 10 times lower than the minimum concentration of Pb(II) authorized in drinking water. The sensor was used also for the determination of Pb(II) in tap, river, and well water samples with only minor loss of sensitivity and recoveries (90±5% to 110±4%). Thanks to the excellent biocompatibility of kaolinite, the sensor was applied for Pb(II) detection in human urine. Recovery in the range 98±8% to 103±6% was obtained when three freshly collected urine samples were spiked with known amounts of Pb(II). These results showed the interesting potential of methoxykaolinite as an electrode modifier for trace-level detection of cations, even in biological samples.







Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information file.
Code Availability
Not applicable.
References
Akanji, S. P., Arotiba, O. A., & Nkosi, D. (2019). Voltammetric determination of Pb (II) ions at a modified kaolinite-carbon paste electrode. Electrocatalysis, 10, 643–652.
Bard, A. J., Faulkner, L. R., & Brisset, J. L. (1983). Electrochimie: principes, méthodes et applications. Masson.
Bouwe, R. G. B., Tonle, I. K., Letaief, S., Ngameni, E., & Detellier, C. (2011). Structural characterisation of 1,10-phenanthroline–montmorillonite intercalation compounds and their application as low-cost electrochemical sensors for Pb(II) detection at the sub-nanomolar level. Applied Clay Science, 52, 258–265.
Cruz, M., Jacobs, H., & Fripiat, J. (1972). The nature of interlayer bonding in kaolin minerals (pp. 35–44). Proceedings of the International Clay Conference, Madrid.
Dedzo, G. K., & Detellier, C. (2016). Functional nanohybrid materials derived from kaolinite. Applied Clay Science, 130, 33–39.
Dedzo, G. K., Letaief, S., & Detellier, C. (2012). Kaolinite–ionic liquid nanohybrid materials as electrochemical sensors for size-selective detection of anions. Journal of Materials Chemistry, 22, 20593–20601.
Dedzo, G. K., Nguelo, B. B., Tonle, I. K., Ngameni, E., & Detellier, C. (2017). Molecular control of the functional and spatial interlayer environment of kaolinite by the grafting of selected pyridinium ionic liquids. Applied Clay Science, 143, 445–451.
Deng, Y., Dixon, J. B., & White, G. N. (2003). Intercalation and surface modification of smectite by two non-ionic surfactants. Clays and Clay Minerals, 51, 150–161.
Detellier, C., & Letaief, S. (2013) Kaolinite–Polymer Nanocomposites. In: Developments in Clay Science (Faïza Bergaya and Gerhard Lagaly, editors). Elsevier, Amsterdam.
Duer, M. J., Rocha, J., & Klinowski, J. (1992). Solid-state NMR studies of the molecular motion in the kaolinite: DMSO intercalate. Journal of the American Chemical Society, 114, 6867–6874.
El Mhammedi, M. A., Achak, M., Bakasse, M., & Chtaini, A. (2009). Electroanalytical method for determination of lead(II) in orange and apple using kaolin modified platinum electrode. Chemosphere, 76, 1130–1134.
Elbokl, T. A., & Detellier, C. (2008). Intercalation of cyclic imides in kaolinite. Journal of Colloid and Interface Science, 323, 338–348.
Elgrishi, N., Rountree, K. J., McCarthy, B. D., Rountree, E. S., Eisenhart, T. T., & Dempsey, J. L. (2018). A Practical Beginner’s Guide to Cyclic Voltammetry. Journal of Chemical Education, 95, 197–206.
Ellis, T. W., & Mirza, A. H. (2010). The refining of secondary lead for use in advanced lead-acid batteries. Journal of Power Sources, 195, 4525–4529.
Fitch, A. (1990). Clay-Modified Electrodes: A Review. Clays and Clay Minerals, 38, 391–400.
Gallo, C., Rizzo, P., & Guerra, G. (2019). Intercalation compounds of a smectite clay with an ammonium salt biocide and their possible use for conservation of cultural heritage. Heliyon, 5, e02991.
Giese, R. F. (1978). The Electrostatic Interlayer Forces of Layer Structure Minerals. Clays and Clay Minerals, 26, 51–57.
Gómez, Y., Fernández, L., Borrás, C., Mostany, J., & Scharifker, B. (2011). Characterization of a carbon paste electrode modified with tripolyphosphate-modified kaolinite clay for the detection of lead. Talanta, 85, 1357–1363.
Gupta, S. S., & Bhattacharyya, K. G. (2012). Adsorption of heavy metals on kaolinite and montmorillonite: a review. Physical Chemistry Chemical Physics, 14, 6698–6723.
Janek, M., Emmerich, K., Heissler, S., & Nüesch, R. (2007). Thermally induced grafting reactions of ethylene glycol and glycerol intercalates of kaolinite. Chemistry of Materials, 19, 684–693.
Jiokeng, S. L. Z., Dongmo, L. M., Ymélé, E., Ngameni, E., & Tonlé, I. K. (2017). Sensitive stripping voltammetry detection of Pb(II) at a glassy carbon electrode modified with an amino-functionalized attapulgite. Sensors and Actuators B: Chemical, 242, 1027–1034.
Komori, Y., Sugahara, Y., & Kuroda, K. (1999). Intercalation of alkylamines and water into kaolinite with methanol kaolinite as an intermediate. Applied Clay Science, 15, 241–252.
Komori, Y., Enoto, H., Takenawa, R., Hayashi, S., Sugahara, Y., & Kuroda, K. (2000). Modification of the interlayer surface of kaolinite with methoxy groups. Langmuir, 16, 5506–5508.
Kuroda, Y., Ito, K., Itabashi, K., & Kuroda, K. (2011). One-step exfoliation of kaolinites and their transformation into nanoscrolls. Langmuir, 27, 2028–2035.
Ledoux, R. L., & White, J. L. (1966). Infrared studies of hydrogen bonding interaction between kaolinite surfaces and intercalated potassium acetate, hydrazine, formamide, and urea. Journal of Colloid and Interface Science, 21, 127–152.
Letaief, S., & Detellier, C. (2008). Ionic liquids-kaolinite nanostructured materials. Intercalation of pyrrolidinium salts. Clays and Clay Minerals, 56, 82–89.
Levallois, P., Barn, P., Valcke, M., Gauvin, D., & Kosatsky, T. (2018). Public health consequences of lead in drinking water. Current Environmental Health Reports, 5, 255–262.
Ma, C., & Eggleton, R. A. (1999). Cation exchange capacity of kaolinite. Clays and Clay Minerals, 47, 174–180.
Maciel, A., Bindewald, E. H., Bergamini, M. F., & Marcolino-Junior, L. H. (2022). Evaluation of titanate nanotubes (TiNTs) as a modifier for the determination of lead (II) by Differential Pulse Adsorptive Stripping Voltammetry (DPAdSV). Analytical Letters, 55, 146–158.
Matusik, J., Gaweł, A., Bielańska, E., Osuch, W., & Bahranowski, K. (2009). The effect of structural order on nanotubes derived from kaolin-group minerals. Clays and Clay Minerals, 57, 452–464.
Mousty, C. (2004). Sensors and biosensors based on clay-modified electrodes – new trends. Applied Clay Science, 27, 159–177.
Murray, H. H. (2000). Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Applied Clay Science, 17, 207–221.
Ngassa, G. B. P., Tonlé, I. K., Walcarius, A., & Ngameni, E. (2014). One-step co-intercalation of cetyltrimethylammonium and thiourea in smectite and application of the organoclay to the sensitive electrochemical detection of Pb(II). Applied Clay Science, 99, 297–305.
O'Connor, D., Hou, D., Ye, J., Zhang, Y., Ok, Y. S., Song, Y., Coulon, F., Peng, T., & Tian, L. (2018). Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications. Environment International, 121, 85–101.
Ogawa, M., Kanaoka, N., & Kuroda, K. (1998). Preparation of Smectite/Dodecyldimethylamine N-oxide Intercalation Compounds. Langmuir, 14, 6969–6973.
Olejnik, S., Aylmore, L. A. G., Posner, A. M., & Quirk, J. P. (1968). Infrared spectra of kaolin mineral-dimethyl sulfoxide complexes. The Journal of Physical Chemistry, 72, 241–249.
Olejnik, S., Posner, A. M., & Quirk, J. P. (1971). The I.R. spectra of interlamellar kaolinite-amide complexes–I. The complexes of formamide, N-methylformamide and dimethylformamide. Clays and Clay Minerals, 19, 83–94.
Salih, F. E., Ouarzane, A., & El Rhazi, M. (2017). Electrochemical detection of lead (II) at bismuth/Poly(1,8-diaminonaphthalene) modified carbon paste electrode. Arabian Journal of Chemistry, 10, 596–603.
Schroeder, P. A., & Erickson, G. (2014). Kaolin: From Ancient Porcelains to Nanocomposites. Elements, 10, 177–182.
Sugahara, Y., Kitano, S., Satokawa, S., Kuroda, K., & Kato, C. (1986). Synthesis of kaolinite-lactam intercalation compounds. Bulletin of the Chemical Society of Japan, 59, 2607–2610.
Tan, D., Yuan, P., Annabi-Bergaya, F., Dong, F., Liu, D., & He, H. (2015a). A comparative study of tubular halloysite and platy kaolinite as carriers for the loading and release of the herbicide amitrole. Applied Clay Science, 114, 190–196.
Tan, D., Yuan, P., Annabi-Bergaya, F., Liu, D., & He, H. (2015b). Methoxy-modified kaolinite as a novel carrier for high-capacity loading and controlled-release of the herbicide amitrole. Scientific Reports, 5, 8870.
Tchoumene, R., Dedzo, G. K., & Ngameni, E. (2018). Preparation of methyl viologen-kaolinite intercalation compound: controlled release and electrochemical applications. ACS Applied Materials & Interfaces, 10, 34534–34542.
Tchoumene, R., Dedzo, G. K., & Ngameni, E. (2022). Intercalation of 1, 2, 4-triazole in methanol modified-kaolinite: Application for copper corrosion inhibition in concentrated sodium chloride aqueous solution. Journal of Solid State Chemistry, 311, 123103.
Tonlé, I. K., Diaco, T., Ngameni, E., & Detellier, C. (2007). Nanohybrid kaolinite-based materials obtained from the interlayer grafting of 3-Aminopropyltriethoxysilane and their potential use as electrochemical sensors. Chemistry of Materials, 19, 6629–6636.
Tonle, I. K., Letaief, S., Ngameni, E., & Detellier, C. (2009). Nanohybrid materials from the grafting of imidazolium cations on the interlayer surfaces of kaolinite. Application as electrode modifier. Journal of Materials Chemistry, 19, 5996–6003.
Tonlé, I. K., Letaief, S., Ngameni, E., Walcarius, A., & Detellier, C. (2011). Square wave voltammetric determination of Lead (II) ions using a carbon paste electrode modified by a thiol-functionalized kaolinite. Electroanalysis, 23, 245–252.
Tunney, J. J., & Detellier, C. (1996). Chemically modified kaolinite. Grafting of methoxy groups on the interlamellar aluminol surface of kaolinite. Journal of Materials Chemistry, 6, 1679–1685.
Wanekaya, A., & Sadik, O. A. (2002). Electrochemical detection of lead using overoxidized polypyrrole films. Journal of Electroanalytical Chemistry, 537, 135–143.
Wang, Y.-H., & Siu, W.-K. (2006). Structure characteristics and mechanical properties of kaolinite soils. I. Surface charges and structural characterizations. Canadian Geotechnical Journal, 43, 587–600.
Wang, Y., Wu, Y., Xie, J., & Hu, X. (2013). Metal–organic framework modified carbon paste electrode for lead sensor. Sensors and Actuators B: Chemical, 177, 1161–1166.
Wang, D., Liu, Q., Cheng, H., Zhang, S., & Zuo, X. (2017). Effect of reaction temperature on intercalation of octyltrimethylammonium chloride into kaolinite. Journal of Thermal Analysis and Calorimetry, 128, 1555–1564.
Wang, R., Ji, W., Huang, L., Guo, L., & Wang, X. (2019). Electrochemical Determination of lead(II) in environmental waters using a sulfydryl-modified covalent organic framework by square wave anodic stripping voltammetry (SWASV). Analytical Letters, 52, 1757–1770.
Watt, G. C. M., Britton, A., Gilmour, H. G., Moore, M. R., Murray, G. D., & Robertson, S. J. (2000). Public health implications of new guidelines for lead in drinking water: a case study in an area with historically high water lead levels. Food and Chemical Toxicology, 38, S73–S79.
Xiong, S., Wang, M., Cai, D., Li, Y., Gu, N., & Wu, Z. (2013). Electrochemical detection of Pb(II) by glassy carbon electrode modified with amine-functionalized magnetite nanoparticles. Analytical Letters, 46, 912–922.
Xu, H., Jin, X., Chen, P., Shao, G., Wang, H., Chen, D., Lu, H., & Zhang, R. (2015). Preparation of kaolinite nanotubes by a solvothermal method. Ceramics International, 41, 6463–6469.
Zazoua, A., Khedimallah, N., & Jaffrezic-Renault, N. (2018). Electrochemical determination of cadmium, lead, and nickel using a polyphenol–polyvinyl chloride–boron-doped diamond electrode. Analytical Letters, 51, 336–347.
Acknowledgments
The authors acknowledge the International Science Program (ISP) – Sweden through funding provided to the African Network of Electroanalytical Chemists (ANEC).
Funding
The work was partially supported by the African Network of Electroanalytical Chemists (ANEC) through the grant offered by the International Science Program (ISP) – Sweden.
Author information
Authors and Affiliations
Contributions
Bruno Boniface Nguelo: Formal analysis, Investigation, Writing - Original Draft Preparation, Writing – Review & Editing, Nganji Sandjon Urtrich: Formal analysis, Investigation, Yami Kamgue Yvana Rusca: Formal analysis, Investigation, Gustave Kenne Dedzo: Conceptualization, Formal analysis, Investigation, Writing – Original Draft Preparation, Writing – Review & Editing, Emmanuel Ngameni: Writing – Review & Editing
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguelo, B.B., Nganji, U.S., Yami, Y.R.K. et al. Electrochemical Study of Methoxykaolinite Interactions with Cations and Application to Trace-Level Detection of Pb(II) in Various Aqueous Media. Clays Clay Miner. 70, 405–416 (2022). https://doi.org/10.1007/s42860-022-00196-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42860-022-00196-3