Abstract
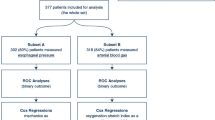
Clinical measurements offer bedside monitoring aiming to minimise unintended over-distension, but have limitations and cannot be predicted for changes in mechanical ventilation (MV) settings and are only available in certain MV modes. This study introduces a non-invasive, real-time over-distension measurement, which is robust, predictable, and more intuitive than current methods. The proposed over-distension measurement, denoted as OD, is compared with the clinically proven stress index (SI). Correlation is analysed via R2 and Spearman rs. The OD safe range corresponding to the unit-less SI safe range (0.95–1.05) is calibrated by sensitivity and specificity test. Validation is fulfilled with 19 acute respiratory distress syndrome (ARDS) patients data (196 cases), including assessment across ARDS severity. Overall correlation between OD and SI yielded R2 = 0.76 and Spearman rs = 0.89. Correlation is higher considering only moderate and severe ARDS patients. Calibration of OD to SI yields a safe range defined: 0 ≤ OD ≤ 0.8 cmH2O. The proposed OD offers an efficient, general, real-time measurement of patient-specific lung mechanics, which is more intuitive and robust than SI. OD eliminates the limitations of SI in MV mode and its less intuitive lung status value. Finally, OD can be accurately predicted for new ventilator settings via its foundation in a validated predictive personalized lung mechanics model. Therefore, OD offers potential clinical value over current clinical methods.





Similar content being viewed by others
References
Tonetti T, Vasques F, Rapetti F, Maiolo G, Collino F, Romitti F, Camporota L, Cressoni M, Cadringher P, Quintel M, Gattinoni L. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med. 2017;5:286. https://doi.org/10.21037/atm.2017.07.08.
Bugedo G, Retamal J, Bruhn A. Does the use of high PEEP levels prevent ventilator-induced lung injury? Rev Bras Terapia Intensiv. 2017;29:231–7. https://doi.org/10.5935/0103-507X.20170032.
Simon P, Girrbach F, Petroff D, Schliewe N, Hempel G, Lange M, Bluth T, Gama de Abreu M, Beda A, Schultz MJ, Pelosi P, Reske AW, Wrigge H, Network* f t P I o t P V, Anesthesiology t C T N o t E S o. Individualized versus fixed positive end-expiratory pressure for intraoperative mechanical ventilation in obese patients: a secondary analysis. Anesthesiology. 2021;134:887–900. https://doi.org/10.1097/ALN.0000000000003762.
Major VJ, Chiew YS, Shaw GM, Chase JG. Biomedical engineer’s guide to the clinical aspects of intensive care mechanical ventilation. Biomed Eng Online. 2018;17:169. https://doi.org/10.1186/s12938-018-0599-9.
Hong CM, Xu D-Z, Lu Q, Cheng Y, Pisarenko V, Doucet D, Brown M, Aisner S, Zhang C, Deitch EA. Low tidal volume and high positive end-expiratory pressure mechanical ventilation results in increased inflammation and ventilator-associated lung injury in normal lungs. Anesth Analg. 2010;110:1652–60.
Gattinoni L, Carlesso E, Brazzi L, Caironi P. Positive end-expiratory pressure. Curr Opin Crit Care. 2010;16:39–44.
Bates JH, Smith BJ. Ventilator-induced lung injury and lung mechanics. Ann Transl Med. 2018;6:378. https://doi.org/10.21037/atm.2018.06.29.
Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–11. https://doi.org/10.1164/ajrccm.164.9.2103121.
Ball L, Sutherasan Y, Pelosi P. Monitoring respiration: what the clinician needs to know. Best Pract Res Clin Anaesthesiol. 2013;27:209–23. https://doi.org/10.1016/j.bpa.2013.06.004.
Zhao Z, Steinmann D, Frerichs I, Guttmann J, Möller K. PEEP titration guided by ventilation homogeneity: a feasibility study using electrical impedance tomography. Crit Care. 2010;14:1–8.
Frerichs I, Amato MB, Van Kaam AH, Tingay DG, Zhao Z, Grychtol B, Bodenstein M, Gagnon H, Böhm SH, Teschner E. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax. 2017;72:83–93.
Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard J-CM, Carvalho CRR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55. https://doi.org/10.1056/NEJMsa1410639.
Goligher EC, Costa ELV, Yarnell CJ, Brochard LJ, Stewart TE, Tomlinson G, Brower RG, Slutsky AS, Amato MPB. Effect of lowering Vt on mortality in acute respiratory distress syndrome varies with respiratory system elastance. Am J Respir Crit Care Med 2021;203(11):1378–1385. https://doi.org/10.1164/rccm.202009-3536OC
Chiew YS, Chase JG, Shaw GM, Sundaresan A, Desaive T. Model-based PEEP optimisation in mechanical ventilation. Biomed Eng Online. 2011;10:111. https://doi.org/10.1186/1475-925X-10-111.
van Drunen EJ, Chiew YS, Pretty C, Shaw GM, Lambermont B, Janssen N, Chase JG, Desaive T. Visualisation of time-varying respiratory system elastance in experimental ARDS animal models. BMC Pulm Med. 2014;14:33. https://doi.org/10.1186/1471-2466-14-33.
Fisher JB, Mammel MC, Coleman JM, Bing DR, Boros SJ. Identifying lung overdistention during mechanical ventilation by using volume-pressure loops. Pediatr Pulmonol. 1988;5:10–4. https://doi.org/10.1002/ppul.1950050104.
Khemani RG, Bart RD, Newth CJL. Respiratory monitoring during mechanical ventilation. Paediatr Child Health. 2007;17:193–201. https://doi.org/10.1016/j.paed.2007.02.006.
Ferrando C, Suárez-Sipmann F, Gutierrez A, Tusman G, Carbonell J, García M, Piqueras L, Compañ D, Flores S, Soro M, Llombart A, Belda FJ. Adjusting tidal volume to stress index in an open lung condition optimizes ventilation and prevents overdistension in an experimental model of lung injury and reduced chest wall compliance. Crit Care. 2015;19:9. https://doi.org/10.1186/s13054-014-0726-3.
Emeriaud G, Newth CJ, Pediatric Acute Lung Injury Consensus Conference G. Monitoring of children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:S86-101. https://doi.org/10.1097/PCC.0000000000000436.
D’Antini D, Huhle R, Herrmann J, Sulemanji DS, Oto J, Raimondo P, Mirabella L, Hemmes SNT, Schultz MJ, Pelosi P, Kaczka DW, Vidal Melo MF, de Abreu MG, Cinnella G, European Society of A, the P V N. Respiratory system mechanics during low versus high positive end-expiratory pressure in open abdominal surgery: a substudy of PROVHILO randomized controlled trial. Anesth Analg. 2018;126:143–9. https://doi.org/10.1213/ANE.0000000000002192.
Carvalho AR, Pacheco SA, de Souza RPV, Bergamini BC, Paula LF, Jandre FC, Giannella-Neto A. Detection of tidal recruitment/overdistension in lung-healthy mechanically ventilated patients under general anesthesia. Anesth Analg. 2013;116:677–84. https://doi.org/10.1213/ANE.0b013e318254230b.
Kano S, Lanteri CJ, Duncan AW, Sly PD. Influence of nonlinearities on estimates of respiratory mechanics using multilinear regression analysis. J Appl Physiol. 1994;77:1185–97. https://doi.org/10.1152/jappl.1994.77.3.1185.
Nève V, Leclerc F, Roque E, Leteurtre S, Riou Y. Overdistension in ventilated children. Crit Care. 2001;5:196–203. https://doi.org/10.1186/cc1023.
Carvalho AR, Spieth PM, Pelosi P, Vidal Melo MF, Koch T, Jandre FC, Giannella-Neto A, de Abreu MG. Ability of dynamic airway pressure curve profile and elastance for positive end-expiratory pressure titration. Intensive Care Med. 2008;34:2291. https://doi.org/10.1007/s00134-008-1301-7.
Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, Mascia L, Pesenti A, Zangrillo A, Gattinoni L. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med. 2012;38:395–403.
Grasso S, Terragni P, Mascia L, Fanelli V, Quintel M, Herrmann P, Hedenstierna G, Slutsky AS, Ranieri VM. Airway pressure-time curve profile (stress index) detects tidal recruitment/hyperinflation in experimental acute lung injury. Crit Care Med. 2004;32:1018–27.
Terragni PP, Filippini C, Slutsky AS, Birocco A, Tenaglia T, Grasso S, Stripoli T, Pasero D, Urbino R, Fanelli V, Faggiano C, Mascia L, Ranieri VM. Accuracy of plateau pressure and stress index to identify injurious ventilation in patients with acute respiratory distress syndrome. Anesthesiology. 2013;119:880–9. https://doi.org/10.1097/ALN.0b013e3182a05bb8.
Esteban A, Anzueto A, AlÍA I, Gordo F, ApezteguÍA C, PÁLizas F, Cide D, Goldwaser R, Soto L, Bugedo G, Rodrigo C, Pimentel J, Raimondi G, Tobin MJ. How is mechanical ventilation employed in the intensive care unit? Am J Respir Crit Care Med. 2000;161:1450–1458. https://doi.org/10.1164/ajrccm.161.5.9902018
Sun Q, Chase JG, Zhou C, Tawhai MH, Knopp JL, Möller K, Shaw GM. Over-distension prediction via hysteresis loop analysis and patient-specific basis functions in a virtual patient model. Comput Biol Med. 2021;141:105022.
Zhou C, Chase JG, Knopp J, Sun Q, Tawhai M, Möller K, Heines SJ, Bergmans DC, Shaw GM, Desaive T. Virtual patients for mechanical ventilation in the intensive care unit. Comput Methods Programs Biomed. 2021;199:105912. https://doi.org/10.1016/j.cmpb.2020.105912.
Sun Q, Zhou C, Chase JG. Parameter updating of a patient-specific lung mechanics model for optimising mechanical ventilation. Biomed Signal Process Control. 2020;60:102003. https://doi.org/10.1016/j.bspc.2020.102003.
Zhou C, Chase JG, Ismail H, Signal MK, Haggers M, Rodgers GW, Pretty C. Silicone phantom validation of breast cancer tumor detection using nominal stiffness identification in digital imaging elasto-tomography (DIET). Biomed Signal Process Control. 2018;39:435–47.
Zhou C, Chase JG, Sun Q, Knopp J. A nonlinear hysteretic model for automated prediction of lung mechanics during mechanical ventilation. IFAC-Pap OnLine. 2020;53:817–22. https://doi.org/10.1016/j.ifacol.2021.04.177.
Zhou C, Chase JG. A new pinched nonlinear hysteretic structural model for automated creation of digital clones in structural health monitoring. Struct Health Monit. 2020;20:101–17. https://doi.org/10.1177/1475921720920641.
Zhou C, Chase JG, Rodgers GW, Tomlinson H, Xu C. Physical parameter identification of structural systems with hysteretic pinching. Comput-Aided Civ Infrastruct Eng. 2015;30:247–62. https://doi.org/10.1111/mice.12108.
Mergoni M, Martelli A, Volpi A, Primavera S, Zuccoli P, Rossi A. Impact of positive end-expiratory pressure on chest wall and lung pressure-volume curve in acute respiratory failure. Am J Respir Crit Care Med. 1997;156:846–54. https://doi.org/10.1164/ajrccm.156.3.9607040.
Mols G, Priebe H-J, Guttmann J. Alveolar recruitment in acute lung injury. BJA Br J Anaesth. 2005;96:156–66. https://doi.org/10.1093/bja/aei299.
Sun X-M, Chen G-Q, Chen K, Wang Y-M, He X, Huang H-W, Luo X-Y, Wang C-M, Shi Z, Xu M, Chen L, Fan E, Zhou J-X. Stress Index can be accurately and reliably assessed by visually inspecting ventilator waveforms. Respir Care. 2018;63:respcare.06151. https://doi.org/10.4187/respcare.06151.
Stahl CA, Möller K, Schumann S, Kuhlen R, Sydow M, Putensen C, Guttmann J. Dynamic versus static respiratory mechanics in acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2006;34:2090–8. https://doi.org/10.1097/01.Ccm.0000227220.67613.0d.
Force* T A D T. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. https://doi.org/10.1001/jama.2012.5669.
Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–8. https://doi.org/10.1213/ane.0000000000002864.
Ratner B. The correlation coefficient: its values range between +1/−1, or do they? J Target Meas Anal Mark. 2009;17:139–42. https://doi.org/10.1057/jt.2009.5.
Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18:91–3. https://doi.org/10.1016/j.tjem.2018.08.001.
Acknowledgements
This work was supported by the NZ Tertiary Education Commission (TEC) fund MedTech CoRE (Centre of Research Excellence; #3705718) and the NZ National Science Challenge 7, Science for Technology and Innovation (2019-S3-CRS). The authors also acknowledge support from the EU H2020 R&I programme (MSCA-RISE-2019 call) under grant agreement #872488—DCPM.
Funding
This work was supported by the NZ Tertiary Education Commission (TEC) fund MedTech CoRE (Centre of Research Excellence; #3705718) and the NZ National Science Challenge 7, Science for Technology and Innovation (2019-S3-CRS). The authors also acknowledge support from the EU H2020 R&I programme (MSCA-RISE-2019 call) under grant agreement #872488—DCPM.
Author information
Authors and Affiliations
Contributions
QS: Conceptualization, Formal analysis, Data curation, Writing—original draft. JGC: Conceptualization, Formal analysis, Writing—review & editing. CZ: Conceptualization, Formal analysis, Writing—review & editing. MHT: Conceptualization. JLK: Conceptualization. KM: Data curation. GMS: Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study data was obtained retrospectively from trials designed in 1999 and conducted between September 2000 and February 2002 in intensive care units of eight German university hospitals. The protocol was approved by the local ethics committee of each participating institution. Informed consent was obtained from the patient or his or her legally authorized representative in accordance with legal and ethical regulations.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Q., Chase, J.G., Zhou, C. et al. Non-invasive over-distension measurements: data driven vs model-based. J Clin Monit Comput 37, 389–398 (2023). https://doi.org/10.1007/s10877-022-00900-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00900-7