Abstract
Introduction
To devise a precise and efficient tool for predicting the individualized risk of acute-phase response (APR) in bisphosphonate (BP)-naive osteoporotic (OP) patients, receiving their first intravenous dose of zoledronate (ZOL).
Methods
The baseline clinical and laboratory data of 475 consecutive BP-naive OP patients, who received their first intravenous dose of ZOL between March 2016 and March 2021 in the Affiliated Kunshan Hospital of Jiangsu University, were chosen for analysis. Univariate and multivariable logistic regression models were generated to establish candidate predictors of APR fever risk, using three distinct fever thresholds, namely, 37.3 °C (model A), 38.0 °C (model B), and 38.5 °C (model C). Next, using predictor regression coefficients, three fever-threshold nomograms were developed. Discrimination, calibration, and clinical usefulness of each predicting models were then assessed using the area under the curve (AUC), calibration curve (CC), and decision curve analysis (DCA). The internal and external model validations were then performed.
Results
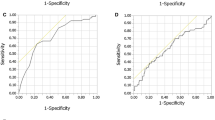
The stable predictors were age, serum 25-hydroxy vitamin D, serum total calcium, and peripheral blood erythrocytes count. These were negatively associated with the APR fever risk. The AUCs of models A, B, and C were 0.828 (95% confidence intervals [CI], 0.782 to 0.874), 0.825 (95% CI, 0.767 to 0.883), and 0.879 (95% CI, 0.824 to 0.934), respectively. Good agreement was observed between the predictions and observations in the CCs of all three nomograms.
Conclusions
This study developed and validated nomogram prediction models that can predict APR fever risk in BP-naive OP patients receiving their first infusion of ZOL.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ding Y, Zeng JC, Yin F, Zhang CL, Zhang Y, Li SX, Liu X, Zhang C, Xue QY, Lin H, Pei FX (2017) Multicenter study on observation of acute-phase responses after infusion of zoledronic acid 5 mg in Chinese women with postmenopausal osteoporosis. Orthop Surg 9(3):284–289. https://doi.org/10.1111/os.12338
Pazianas M, van der Geest S, Miller P (2014) Bisphosphonates and bone quality. Bonekey Rep 3:529. https://doi.org/10.1038/bonekey.2014.24
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81(8):1013–1022. https://doi.org/10.4065/81.8.1013
Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, Widmer A, Devogelaer JP, Kaufman JM, Jaeger P, Body JJ, Brandi ML, Broell J, Di Micco R, Genazzani AR, Felsenberg D, Happ J, Hooper MJ, Ittner J, Leb G, Mallmin H, Murray T, Ortolani S, Rubinacci A, Saaf M, Samsioe G, Verbruggen L, Meunier PJ (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346(9):653–661. https://doi.org/10.1056/NEJMoa011807
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356(18):1809–1822. https://doi.org/10.1056/NEJMoa067312
Curtis JR, Yun H, Matthews R, Saag KG, Delzell E (2012) Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res (Hoboken) 64(7):1054–1060. https://doi.org/10.1002/acr.21638
Modi A, Sajjan S, Insinga R, Weaver J, Lewiecki EM, Harris ST (2017) Frequency of discontinuation of injectable osteoporosis therapies in US patients over 2 years. Osteoporos Int 28(4):1355–1363. https://doi.org/10.1007/s00198-016-3886-y
Lee YK, Nho JH, Ha YC, Koo KH (2012) Persistence with intravenous zoledronate in elderly patients with osteoporosis. Osteoporos Int 23(9):2329–2333. https://doi.org/10.1007/s00198-011-1881-x
Ziller V, Kostev K, Kyvernitakis I, Boeckhoff J, Hadji P (2012) Persistence and compliance of medications used in the treatment of osteoporosis–analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther 50(5):315–322. https://doi.org/10.5414/cp201632
Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM (2010) Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 95(9):4380–4387. https://doi.org/10.1210/jc.2010-0597
Adami S, Bhalla AK, Dorizzi R, Montesanti F, Rosini S, Salvagno G, Lo Cascio V (1987) The acute-phase response after bisphosphonate administration. Calcif Tissue Int 41(6):326–331. https://doi.org/10.1007/bf02556671
Nakamura T, Fukunaga M, Nakano T, Kishimoto H, Ito M, Hagino H, Sone T, Taguchi A, Tanaka S, Ohashi M, Ota Y, Shiraki M (2017) Efficacy and safety of once-yearly zoledronic acid in Japanese patients with primary osteoporosis: two-year results from a randomized placebo-controlled double-blind study (ZOledroNate treatment in Efficacy to osteoporosis; ZONE study). Osteoporos Int 28(1):389–398. https://doi.org/10.1007/s00198-016-3736-y
Bertoldo F, Pancheri S, Zenari S, Boldini S, Giovanazzi B, Zanatta M, Valenti MT, Dalle Carbonare L, Lo Cascio V (2010) Serum 25-hydroxyvitamin D levels modulate the acute-phase response associated with the first nitrogen-containing bisphosphonate infusion. J Bone Miner Res 25(3):447–454. https://doi.org/10.1359/jbmr.090819
Shiraki M, Kuroda T, Takeuchi Y, Sugimoto T, Tanaka S, Suzuki H, Hiraishi K, Nakamura T (2021) Acute phase reactions after intravenous infusion of zoledronic acid in Japanese patients with osteoporosis: sub-analyses of the phase III ZONE study. Calcif Tissue Int 109(6):666–674. https://doi.org/10.1007/s00223-021-00884-7
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB (2020) American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract 26(Suppl 1):1–46. https://doi.org/10.4158/gl-2020-0524suppl
Collins GS, Reitsma JB, Altman DG, Moons KG (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol 68(2):134–143. https://doi.org/10.1016/j.jclinepi.2014.11.010
Wark JD, Bensen W, Recknor C, Ryabitseva O, Chiodo J 3rd, Mesenbrink P, de Villiers TJ (2012) Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int 23(2):503–512. https://doi.org/10.1007/s00198-011-1563-8
Okimoto N, Sakai A, Yoshioka T, Kobayashi T, Asano K, Akahoshi S, Ishikura T, Fukuhara S, Fuse Y, Mizuno T, Katae Y, Matsumoto H, Ogawa T, Nishida S, Ikeda S, Menuki K, Saito J, Okazaki Y, Mizuno N, Fujiwara S (2020) Efficacy of non-steroidal anti-inflammatory drugs on zoledronic acid-induced acute-phase reactions: randomized, open-label, Japanese OZ study. J Bone Miner Metab 38(2):230–239. https://doi.org/10.1007/s00774-019-01050-8
Iasonos A, Schrag D, Raj GV, Panageas KS (2008) How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26(8):1364–1370. https://doi.org/10.1200/jco.2007.12.9791
Silva TB, Oliveira CZ, Faria EF, Mauad EC, Syrjänen KJ, Carvalho AL (2015) Development and validation of a nomogram to estimate the risk of prostate cancer in Brazil. Anticancer Res 35(5):2881–2886
Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, McGinn T, Guyatt G (2017) Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA 318(14):1377–1384. https://doi.org/10.1001/jama.2017.12126
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77. https://doi.org/10.1186/1471-2105-12-77
Vickers AJ, Cronin AM, Elkin EB, Gonen M (2008) Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak 8:53. https://doi.org/10.1186/1472-6947-8-53
Sauty A, Pecherstorfer M, Zimmer-Roth I, Fioroni P, Juillerat L, Markert M, Ludwig H, Leuenberger P, Burckhardt P, Thiebaud D (1996) Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone 18(2):133–139. https://doi.org/10.1016/8756-3282(95)00448-3
Scheller EL, Hankenson KD, Reuben JS, Krebsbach PH (2011) Zoledronic acid inhibits macrophage SOCS3 expression and enhances cytokine production. J Cell Biochem 112(11):3364–3372. https://doi.org/10.1002/jcb.23267
Thiébaud D, Sauty A, Burckhardt P, Leuenberger P, Sitzler L, Green JR, Kandra A, Zieschang J, Ibarra de Palacios P (1997) An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int 61(5):386–392. https://doi.org/10.1007/s002239900353
Dicuonzo G, Vincenzi B, Santini D, Avvisati G, Rocci L, Battistoni F, Gavasci M, Borzomati D, Coppola R, Tonini G (2003) Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interferon Cytokine Res 23(11):649–654. https://doi.org/10.1089/107999003322558782
van Beek E, Pieterman E, Cohen L, Löwik C, Papapoulos S (1999) Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun 264(1):108–111. https://doi.org/10.1006/bbrc.1999.1499
Roelofs AJ, Jauhiainen M, Mönkkönen H, Rogers MJ, Mönkkönen J, Thompson K (2009) Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol 144(2):245–250. https://doi.org/10.1111/j.1365-2141.2008.07435.x
Popp AW, Senn R, Curkovic I, Senn C, Buffat H, Popp PF, Lippuner K (2017) Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int 28(6):1995–2002. https://doi.org/10.1007/s00198-017-3992-5
Chen J, Yu L, Chen L, Wu X, Tang P, Yin J, Jiang T, Yin G, Fan J (2017) Surgical trauma and low-dose methylprednisolone modulate the severity of the acute-phase response induced by zoledronic acid infusion. Exp Ther Med 14(2):1802–1808. https://doi.org/10.3892/etm.2017.4646
Takada J, Iba K, Yamamoto O, Dohke T, Saito A, Yamamura M, Takebayashi T, Akatsuka T, Yamashita T (2021) Early adverse events after the first administration of zoledronic acid in Japanese patients with osteoporosis. J Bone Miner Metab 39(5):903–910. https://doi.org/10.1007/s00774-021-01231-4
Chen L, Cencioni MT, Angelini DF, Borsellino G, Battistini L, Brosnan CF (2005) Transcriptional profiling of gamma delta T cells identifies a role for vitamin D in the immunoregulation of the V gamma 9V delta 2 response to phosphate-containing ligands. J Immunol 174(10):6144–6152. https://doi.org/10.4049/jimmunol.174.10.6144
Davies M, Heys SE, Selby PL, Berry JL, Mawer EB (1997) Increased catabolism of 25-hydroxyvitamin D in patients with partial gastrectomy and elevated 1,25-dihydroxyvitamin D levels. Implications for metabolic bone disease. J Clin Endocrinol Metab 82(1):209–212. https://doi.org/10.1210/jcem.82.1.3644
Clements MR, Johnson L, Fraser DR (1987) A new mechanism for induced vitamin D deficiency in calcium deprivation. Nature 325(6099):62–65. https://doi.org/10.1038/325062a0
Horwitz MJ, Tedesco MB, Sereika SM, Hollis BW, Garcia-Ocaña A, Stewart AF (2003) Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1–36) [hPTHrP-(1–36)] versus hPTH-(1–34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab 88(4):1603–1609. https://doi.org/10.1210/jc.2002-020773
Burnett-Bowie SM, Henao MP, Dere ME, Lee H, Leder BZ (2009) Effects of hPTH(1–34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res 24(10):1681–1685. https://doi.org/10.1359/jbmr.090406
Cosman F, Nieves J, Morgan D, Shen V, Sherwood D, Parisien M, Lindsay R (1999) Parathyroid hormone secretory response to EDTA-induced hypocalcemia in black and white premenopausal women. Calcif Tissue Int 65(4):257–261. https://doi.org/10.1007/s002239900694
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281. https://doi.org/10.1056/NEJMra070553
Feyen JH, Elford P, Di Padova FE, Trechsel U (1989) Interleukin-6 is produced by bone and modulated by parathyroid hormone. J Bone Miner Res 4(4):633–638. https://doi.org/10.1002/jbmr.5650040422
Greenfield EM, Horowitz MC, Lavish SA (1996) Stimulation by parathyroid hormone of interleukin-6 and leukemia inhibitory factor expression in osteoblasts is an immediate-early gene response induced by cAMP signal transduction. J Biol Chem 271(18):10984–10989. https://doi.org/10.1074/jbc.271.18.10984
Grey A, Mitnick MA, Shapses S, Ellison A, Gundberg C, Insogna K (1996) Circulating levels of interleukin-6 and tumor necrosis factor-alpha are elevated in primary hyperparathyroidism and correlate with markers of bone resorption–a clinical research center study. J Clin Endocrinol Metab 81(10):3450–3454. https://doi.org/10.1210/jcem.81.10.8855783
Montalbán C, García-Unzueta MT, De Francisco AL, Amado JA (1999) Serum interleukin-6 in renal osteodystrophy: relationship with serum PTH and bone remodeling markers. Horm Metab Res 31(1):14–17. https://doi.org/10.1055/s-2007-978689
Ganz T (2019) Anemia of Inflammation. N Engl J Med 381(12):1148–1157. https://doi.org/10.1056/NEJMra1804281
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340(6):448–454. https://doi.org/10.1056/nejm199902113400607
Desborough JP (2000) The stress response to trauma and surgery. Br J Anaesth 85(1):109–117. https://doi.org/10.1093/bja/85.1.109
Candore G, Balistreri CR, Colonna-Romano G, Lio D, Listì F, Vasto S, Caruso C (2010) Gender-related immune-inflammatory factors, age-related diseases, and longevity. Rejuvenation Res 13(2–3):292–297. https://doi.org/10.1089/rej.2009.0942
Maggio M, Blackford A, Taub D, Carducci M, Ble A, Metter EJ, Braga-Basaria M, Dobs A, Basaria S (2006) Circulating inflammatory cytokine expression in men with prostate cancer undergoing androgen deprivation therapy. J Androl 27(6):725–728. https://doi.org/10.2164/jandrol.106.000141
Attivi D, Kosmalski G, Zeghmouli C, Gibaud S (2014) Effect of intravenous hydration in patients receiving bisphosphonate therapy. Int J Clin Pharm 36(6):1277–1281. https://doi.org/10.1007/s11096-014-9994-x
Black DM, Reid IR, Napoli N, Ewing SK, Shiraki M, Nakamura T, Takeuchi Y, Schafer AL, Kim TY, Cauley JA (2021) The interaction of acute-phase reaction and efficacy for osteoporosis after zoledronic acid: HORIZON pivotal fracture trial. J Bone Miner Res. https://doi.org/10.1002/jbmr.4434
Funding
The study was supported by the National Natural Science Foundation of China (CN) (82172441), Scientific Research Project of Gusu School of Nanjing Medical University (CN) (GSKY20210244), Clinical Medical Science and Technology Development Fund of Jiangsu University (CN) (JLY2021048), and Suzhou Key Clinical Diagnosis and Treatment Technology Project (CN) (LCZX202024).
Author information
Authors and Affiliations
Contributions
Ke Lu: Wrote the manuscript. Collected and analyzed the data. Qin Shi: Designed the study. Ya-qin Gong: collected and managed the data. Jia-wei Shao: collected and analyzed the data. Chong Li: designed the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This is a retrospective observational study, based on medical data from a clinical registry. This work is registered in the Chinese Clinical Trial Registry (ChiCTR2000036375). In addition, we received ethical approval from the Affiliated Kunshan Hospital of Jiangsu University (approval No. 2020–03-046-K01), and the study was compliant with the Declaration of Helsinki. All participants provided written informed consent for participation and data collection.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, K., Shi, Q., Gong, Yq. et al. Predicting the acute-phase response fever risk in bisphosphonate-naive osteoporotic patients receiving their first dose of zoledronate. Osteoporos Int 33, 2381–2396 (2022). https://doi.org/10.1007/s00198-022-06493-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06493-w