Abstract
The ε4 allele of the apolipoprotein E (APOE) gene, a genetic risk factor for Alzheimer’s disease, is abundantly expressed in both the brain and periphery. Here, we present evidence that peripheral apoE isoforms, separated from those in the brain by the blood–brain barrier, differentially impact Alzheimer’s disease pathogenesis and cognition. To evaluate the function of peripheral apoE, we developed conditional mouse models expressing human APOE3 or APOE4 in the liver with no detectable apoE in the brain. Liver-expressed apoE4 compromised synaptic plasticity and cognition by impairing cerebrovascular functions. Plasma proteome profiling revealed apoE isoform-dependent functional pathways highlighting cell adhesion, lipoprotein metabolism and complement activation. ApoE3 plasma from young mice improved cognition and reduced vessel-associated gliosis when transfused into aged mice, whereas apoE4 compromised the beneficial effects of young plasma. A human induced pluripotent stem cell-derived endothelial cell model recapitulated the plasma apoE isoform-specific effect on endothelial integrity, further supporting a vascular-related mechanism. Upon breeding with amyloid model mice, liver-expressed apoE4 exacerbated brain amyloid pathology, whereas apoE3 reduced it. Our findings demonstrate pathogenic effects of peripheral apoE4, providing a strong rationale for targeting peripheral apoE to treat Alzheimer’s disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets generated and/or analyzed in the current study are attached. The bulk RNA-seq data and scRNA-seq data that support the findings of this study are deposited in the Gene Expression Omnibus repository under accession numbers GSE125501 (bulk RNA-seq) and GSE206204 (scRNA-seq). Source data are provided with this paper.
References
Guo, T. et al. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 15, 40 (2020).
de la Torre, J. C. Alzheimer disease as a vascular disorder: nosological evidence. Stroke 33, 1152–1162 (2002).
Whitmer, R. A., Karter, A. J., Yaffe, K., Quesenberry, C. P. Jr. & Selby, J. V. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 301, 1565–1572 (2009).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Di Marco, L. Y., Farkas, E., Martin, C., Venneri, A. & Frangi, A. F. Is vasomotion in cerebral arteries impaired in Alzheimer’s disease? J. Alzheimers Dis. 46, 35–53 (2015).
Nguyen, B., Bix, G. & Yao, Y. Basal lamina changes in neurodegenerative disorders. Mol. Neurodegener. 16, 81 (2021).
Farrer, L. A. et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. a meta-analysis. apoe and Alzheimer disease meta analysis consortium. JAMA 278, 1349–1356 (1997).
Hyman, B. T. et al. Apolipoprotein E and cognitive change in an elderly population. Ann. Neurol. 40, 55–66 (1996).
Castellano, J. M. et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci. Transl. Med 3, 89ra57 (2011).
Reiman, E. M. et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl Acad. Sci. USA 106, 6820–6825 (2009).
Liu, C. C., Kanekiyo, T., Xu, H. & Bu, G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118 (2013).
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012).
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C. & Bu, G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518 (2019).
Williams, T., Borchelt, D. R. & Chakrabarty, P. Therapeutic approaches targeting Apolipoprotein E function in Alzheimer’s disease. Mol. Neurodegener. 15, 8 (2020).
Linton, M. F. et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest. 88, 270–281 (1991).
Lane-Donovan, C. et al. Genetic restoration of plasma ApoE improves cognition and partially restores synaptic defects in ApoE-deficient mice. J. Neurosci. 36, 10141–10150 (2016).
Martinez-Morillo, E. et al. Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer’s disease patients and controls. Acta Neuropathol. 127, 633–643 (2014).
Tai, L. M. et al. The role of APOE in cerebrovascular dysfunction. Acta Neuropathol. 131, 709–723 (2016).
Haan, M. N., Shemanski, L., Jagust, W. J., Manolio, T. A. & Kuller, L. The role of APOE ∊4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA 282, 40–46 (1999).
Liu, C. C. et al. ApoE4 accelerates early seeding of amyloid pathology. Neuron 96, 1024–1032 (2017).
Davalos, D. et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun. 3, 1227 (2012).
Fukuda, E. et al. Down-regulation of protocadherin-alpha A isoforms in mice changes contextual fear conditioning and spatial working memory. Eur. J. Neurosci. 28, 1362–1376 (2008).
Siddiqui, M. R., Mayanil, C. S., Kim, K. S. & Tomita, T. Angiopoietin-1 regulates brain endothelial permeability through PTPN-2 mediated tyrosine dephosphorylation of occludin. PLoS ONE 10, e0130857 (2015).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Sheng, J. & Xu, Z. Three decades of research on angiogenin: a review and perspective. Acta Biochim. Biophys. Sin. 48, 399–410 (2016).
Subramanian, A. et al. Gene-set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Baeten, K. M. & Akassoglou, K. Extracellular matrix and matrix receptors in blood–brain barrier formation and stroke. Dev. Neurobiol. 71, 1018–1039 (2011).
Song, W. M. & Zhang, B. Multiscale embedded gene coexpression network analysis. PLoS Comput Biol. 11, e1004574 (2015).
Pearson-Leary, J. et al. Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl. Psychiatry 7, e1160 (2017).
Piskunov, A. et al. Chronic combined stress induces selective and long-lasting inflammatory response evoked by changes in corticosterone accumulation and signaling in rat hippocampus. Metab. Brain Dis. 31, 445–454 (2016).
Nelimarkka, L. et al. Decorin is produced by capillary endothelial cells in inflammation-associated angiogenesis. Am. J. Pathol. 158, 345–353 (2001).
Vanlandewijck, M. et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018).
Chen, M. B. et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep. 30, 4418–4432 (2020).
Ceradini, D. J. et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med 10, 858–864 (2004).
Noubade, R. et al. von-Willebrand factor influences blood–brain barrier permeability and brain inflammation in experimental allergic encephalomyelitis. Am. J. Pathol. 173, 892–900 (2008).
Fan, D. & Kassiri, Z. Biology of tissue inhibitor of metalloproteinase 3 (TIMP3), and its therapeutic implications in cardiovascular pathology. Front Physiol. 11, 661 (2020).
Dewing, J. M., Carare, R. O., Lotery, A. J. & Ratnayaka, J. A. The diverse roles of TIMP3-3: insights into degenerative diseases of the senescent retina and brain. Cells 9, 39 (2019).
Eckhouse, S. R. et al. Local hydrogel release of recombinant TIMP-3 attenuates adverse left ventricular remodeling after experimental myocardial infarction. Sci. Transl. Med 6, 223ra221 (2014).
Middeldorp, J. et al. Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol. 73, 1325–1333 (2016).
Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med 20, 659–663 (2014).
Jankowsky, J. L. et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 13, 159–170 (2004).
Liu, C. C. et al. Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-beta clearance and aggregation in Alzheimer’s disease. Sci. Transl. Med. 8, 332ra344 (2016).
Hawkes, C. A. et al. Disruption of arterial perivascular drainage of amyloid-beta from the brains of mice expressing the human APOE ε4 allele. PLoS ONE 7, e41636 (2012).
Holtzman, D. M. et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 97, 2892–2897 (2000).
Ulrich, J. D. et al. ApoE facilitates the microglial response to amyloid plaque pathology. J. Exp. Med. 215, 1047–1058 (2018).
Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405 (2015).
Bulgart, H. R., Neczypor, E. W., Wold, L. E. & Mackos, A. R. Microbial involvement in Alzheimer disease development and progression. Mol. Neurodegener. 15, 42 (2020).
Pluvinage, J. V. & Wyss-Coray, T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat. Rev. Neurosci. 21, 93–102 (2020).
Boada, M. et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: primary results of the AMBAR study. Alzheimers Dement. 16, 1412–1425 (2020).
Nielsen, H. M. et al. Peripheral apoE isoform levels in cognitively normal APOE ε3/ε4 individuals are associated with regional gray matter volume and cerebral glucose metabolism. Alzheimers Res. Ther. 9, 5 (2017).
Stohr, R. et al. Loss of TIMP3 exacerbates atherosclerosis in ApoE null mice. Atherosclerosis 235, 438–443 (2014).
Thevenard, J. et al. Low-density lipoprotein receptor-related protein-1 mediates endocytic clearance of tissue inhibitor of metalloproteinases-1 and promotes its cytokine-like activities. PLoS ONE 9, e103839 (2014).
Markiewski, M. M. & Lambris, J. D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 171, 715–727 (2007).
Nation, D. A. et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276 (2019).
Hussain, B., Fang, C. & Chang, J. Blood–brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia. Front Neurosci. 15, 688090 (2021).
Merlini, M. et al. Fibrinogen induces microglia-mediated spine elimination and cognitive impairment in an Alzheimer’s disease model. Neuron 101, 1099–1108 (2019).
Cortes-Canteli, M. et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease. Neuron 66, 695–709 (2010).
Tan, Y. et al. 7,8-Dihydroxyflavone ameliorates cognitive impairment by inhibiting expression of tau pathology in ApoE-knockout mice. Front. Aging Neurosci. 8, 287 (2016).
Hartman, R. E. et al. Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer’s-like neuropathology. Exp. Neurol. 170, 326–344 (2001).
Kim, J. et al. Anti-apoE immunotherapy inhibits amyloid accumulation in a transgenic mouse model of Aβ amyloidosis. J. Exp. Med. 209, 2149–2156 (2012).
Liao, F. et al. Anti-ApoE antibody given after plaque onset decreases Aβ accumulation and improves brain function in a mouse model of Aβ amyloidosis. J. Neurosci. 34, 7281–7292 (2014).
Huynh, T. V. et al. Lack of hepatic apoE does not influence early Aβ deposition: observations from a new APOE knock-in model. Mol. Neurodegener. 14, 37 (2019).
Miyazaki, S., Miyazaki, T., Tashiro, F., Yamato, E. & Miyazaki, J. Development of a single-cassette system for spatiotemporal gene regulation in mice. Biochem. Biophys. Res. Commun. 338, 1083–1088 (2005).
Chakrabarty, P. et al. IFN-γ promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J. Immunol. 184, 5333–5343 (2010).
Liu, C. C. et al. APOE3-Jacksonville (V236E) variant reduces self-aggregation and risk of dementia. Sci. Transl. Med. 13, eabc9375 (2021).
Drew, P. J., Blinder, P., Cauwenberghs, G., Shih, A. Y. & Kleinfeld, D. Rapid determination of particle velocity from space-time images using the Radon transform. J. Comput. Neurosci. 29, 5–11 (2010).
Zhao, J. et al. Retinoic acid isomers facilitate apolipoprotein E production and lipidation in astrocytes through the retinoid X receptor/retinoic acid receptor pathway. J. Biol. Chem. 289, 11282–11292 (2014).
Liu, C. C. et al. Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer’s disease. Neuron 84, 63–77 (2014).
Rogers, J. T. et al. Subacute ibuprofen treatment rescues the synaptic and cognitive deficits in advanced-aged mice. Neurobiol. Aging 53, 112–121 (2017).
Liu, C. C. et al. Tau and apolipoprotein E modulate cerebrovascular tight junction integrity independent of cerebral amyloid angiopathy in Alzheimer’s disease. Alzheimers Dement. 16, 1372–1383 (2020).
Kalari, K. R. et al. MAP-RSeq: Mayo analysis pipeline for RNA sequencing. BMC Bioinformatics 15, 224 (2014).
Hansen, K. D., Irizarry, R. A. & Wu, Z. Removing technical variability in RNA-seq data using conditional quantile normalization. Biostatistics 13, 204–216 (2012).
Piedrahita, J. A., Zhang, S. H., Hagaman, J. R., Oliver, P. M. & Maeda, N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl Acad. Sci. USA 89, 4471–4475 (1992).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008).
Yamazaki, A. et al. Preparation of single-cell suspensions enriched in mouse brain vascular cells for single-cell RNA sequencing. STAR Protoc. 2, 100715 (2021).
Yamazaki, Y. et al. Vascular ApoE4 impairs behavior by modulating gliovascular function. Neuron 109, 438–447 (2021).
Heo, J. et al. Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology 44, 1478–1486 (2006).
Hu, J. et al. Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Aβ metabolism in apoE4-targeted replacement mice. Mol. Neurodegener. 10, 6 (2015).
Dey, K. K. et al. Deep undepleted human serum proteome profiling toward biomarker discovery for Alzheimer’s disease. Clin. Proteomics 16, 16 (2019).
Wang, H. et al. Integrated analysis of ultra-deep proteomes in cortex, cerebrospinal fluid and serum reveals a mitochondrial signature in Alzheimer’s disease. Mol. Neurodegener. 15, 43 (2020).
Xu, P., Duong, D. M. & Peng, J. M. Systematical optimization of reverse-phase chromatography for shotgun proteomics. J. Proteome Res. 8, 3944–3950 (2009).
Wang, Z. et al. 27-plex tandem mass tag mass spectrometry for profiling brain proteome in Alzheimer’s disease. Anal. Chem. 92, 7162–7170 (2020).
Wang, Z. et al. High-throughput and deep-proteome profiling by 16-plex tandem mass tag labeling coupled with two-dimensional chromatography and mass spectrometry. J. Vis. Exp. e61684 (2020).
Wang, X. et al. JUMP: a tag-based database search tool for peptide identification with high sensitivity and accuracy. Mol. Cell. Proteomics 13, 3663–3673 (2014).
Peng, J., Elias, J. E., Thoreen, C. C., Licklider, L. J. & Gygi, S. P. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC–MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2, 43–50 (2003).
Niu, M. et al. Extensive peptide fractionation and Y1 ion-based interference detection method for enabling accurate quantification by isobaric labeling and mass spectrometry. Anal. Chem. 89, 2956–2963 (2017).
Qian, T. et al. Directed differentiation of human pluripotent stem cells to blood–brain barrier endothelial cells. Sci. Adv. 3, e1701679 (2017).
Acknowledgements
We are grateful to D. Dickson, M. C. Casey, L. Rousseau, V. Phillips and A. Librero at Mayo Clinic Histology Core for the immunohistochemical analyses. We thank M. Heckman at Mayo Clinic for the consultation of the statistical analysis. We thank M. Li and J. Snyder from the Hope Center Viral Vectors Core at the Washington University School of Medicine for producing the AAV viruses. This work was supported by NIH grants RF1AG046205, R37AG027924, RF1AG057181 and U19AG069701 (to G.B.) and a grant from the Cure Alzheimer’s Fund (to G.B.), NIH grants R21AG057981 and R01AG62110 (to C.-C.L.), a grant from the BrightFocus Foundation (to C.-C.L.) and NIH grants U01AG046170, RF1AG054014 and U01AG052411 (to B.Z.).
Author information
Authors and Affiliations
Contributions
C.-C.L., Y.F. and G.B. conceived and designed the project. C.-C.L. and G.B. wrote the paper. C.-C.L., J.Z., Y.F., N.W., L.J., Z.T., L.F., Y.A.M. and A.K. executed the experiments and analyzed the data. Yuanxin Chen and B.K. performed two-photon imaging and provided knowledge support for two-photon experiments. Y.I. performed human iPSC-derived brain endothelial cell-related experiments. J.Z, J.K. and Y.F. performed young plasma injection experiments. J.Z., T.Z., F.S., Y.F., J.L., N.W., N.Z., Y.I., S.J., S.V.D., C.L.R., L.A.K. and C.-C.L. performed immunofluorescence staining, immunohistological and biochemical analyses. S.V.D., L.B. and P.S. performed RNAScope experiments and provided technical support. Z.W. and J.P. performed plasma proteomics analysis and provided knowledge support. D.K. and X.W. constructed regulatory networks between plasma proteomics and scRNA-seq data. Z.L., S.V.D. and J.Z. carefully proofread and edited the manuscript. Y.Y., A.Y., Y.A.M., L.F. and C.-C.L. performed scRNA-seq and bulk RNA-seq experiments. Y.R., Z.Q., M.W., B.Z. and Y.A. performed bioinformatics analysis and provided knowledge for bulk RNA-seq and scRNA-seq analyses. W.Q. and J.R. performed electrophysiological analyses. C.-C.L., N.Z., L.J., C.L., Yixing Chen and J.K. contributed to the animal maintenance, genotyping and tissue harvest. T.K., J.D.F., B.Z., Y.A., B.K. and G.B. contributed the knowledge and/or the expertise in the project. All authors have read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
G.B. consults for SciNeuro and Lexeo, is on the scientific advisory board for Kisbee and has consulted for AbbVie, E-Scape, Eisai and Vida Ventures. All other authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Liver-expressed apoE3 restores peripheral lipid profiles.
a, EGFP, a surrogate of apoE expression, was detected in liver of iE3/Cre+ but not iE3/Cre−mice. Similar results were observed in at least three independent experiments. Scale bar, 50 µm. b-d, ApoE levels in the liver, plasma, and brain of iE3/Cre−, iE3/Cre+ (Cre−, n = 10; Cre+, n = 15), and apoE3-TR (n = 8) mice were examined by ELISA. **, P < 0.0001. e, The apoE was not detected by Western blotting in the brains of iE3/Cre− or iE3/Cre+ mice compared to apoE3-TR mice (n = 4/group). **, P < 0.0001. f, The RNA levels for apoE in the brains of iE3/Cre, apoE3-TR, iE4/Cre, and apoE4-TR mice (n = 8/group) were examined by real-time PCR. **, P < 0.0001. g, Total cholesterol and triglyceride levels in the plasma of iE3/Cre− or iE3/Cre+ mice (Cre−, n = 9; Cre+, n = 11) and apoE3-TR mice (n = 8) were examined. Cholesterol: **, P < 0.0001. Triglyceride: Cre− vs. Cre+: **, P = 0.002; Cre− vs. E3: **, P = 0.0002. h, Distribution of plasma lipoprotein cholesterol in iE3/Cre− or iE3/Cre+ mice (n = 3/group) at 12 months of age. Pooled plasma from apoE3-TR mice (n = 3/group) was included as control. Data represent mean ± s.e.m. One-way analysis of variance (ANOVA) with Tukey’s post-hoc test was used for statistical analyses. N.S., not significant.
Extended Data Fig. 2 Expression of apoE4 in the liver leads to compromised tight junction integrity in the brain.
a, Brain sections from iE4/Cre− or iE4/Cre+ mice (Cre−, n = 11; Cre+, n = 14) at 12-13 months of age were immunostained with anti-claudin-5 (CLDN5; green) and anti-Glut1 (red) antibodies. The total Glut1 signals and the CLDN5 signals normalized against Glut1 were quantified. *, P = 0.024. Scale bar, 25 µm. b, Brain sections from the iE4/Cre− or the iE4/Cre+ mice (Cre−, n = 12; Cre+, n = 14) at 12-13 months of age were immunostained with anti-ZO1 (green) and anti-Glut1 (red) antibodies. The total Glut1 signals and the ZO1 signals normalized against Glut1 were quantified. *, P = 0.038. Scale bar, 25 µm. c, Brain sections from iE4/Cre− or iE4/Cre+ mice (Cre−, n = 11; Cre+, n = 13) at 12-13 months of age were immunostained with anti-IgG (green) and anti-Glut1 (red) antibodies. The total Glut1 signals and the IgG signals normalized against Glut1 were quantified. *, P = 0.047. Scale bar, 10 µm. Data represent mean ± s.e.m. d, Brain sections from iE4/Cre− or iE4/Cre+ mice (Cre−, n = 8; Cre+, n = 9) at 12-13 months of age were immunostained with anti-fibrinogen (green) and anti-Glut1 (red) antibodies. The total Glut1 signals and the fibrinogen signals normalized against Glut1 were quantified. *, P = 0.034. Scale bar, 10 µm. Data represent mean ± s.e.m. e, Brain sections from the iE4/Cre− or iE4/Cre+ mice (Cre−, n = 8; Cre+, n = 9) at 12-13 months of age were immunostained with anti-CD13 (red) for pericytes and anti-Glut1 (green) antibodies. The total Glut1 signals and the CD13 signals normalized against Glut1 were quantified. Scale bar, 25 µm. Data represent mean ± s.e.m. N.S., not significant, two-tailed Student’s t-test.
Extended Data Fig. 3 Cerebral vascular function in mice expressing apoE3 or apoE4 in the liver by two-photon imaging.
a-d, The BBB integrity, CBF, and vasomotion in the arterioles of iE4/Cre− and iE4/Cre+ mice were assessed by two-photon imaging. a, CBF in the cerebral veins and capillaries of iE4/Cre− or iE4/Cre+ mice at 6-7 months of age were measured. Each data point represents blood vessel-related measurement with various number of measurements in each animal from 5 animals per genotype. b, c, Vessel branch densities in the cerebral arterioles of iE4/Cre− or iE4/Cre+ mice (Cre−, n = 3; Cre+, n = 3) at 1.5~2 months of age were assessed. Scale bar, 50 µm. d, CBF in the cerebral arterioles of iE4/Cre− or iE4/Cre+ mice at young age (1.5~2-month-old) was examined. e−j, BBB permeability, CBF, and vasomotion in the arterioles of iE3/Cre− and iE3/Cre+ mice (Cre−, n = 3; Cre+, n = 3) at 6-7 months of age were assessed by two-photon imaging. Each data point represents blood vessel-related measurement with various number of measurements in each animal from 3 animals per genotype. e, CBF in the cerebral arterioles of iE3/Cre− and iE3/Cre+ mice was examined. f, BBB integrity was examined after intravenous injection of Texas Red conjugated dextran (40 kDa) for 30 mins. Scale bar, 100 µm. g, Fractions of BBB leakage over time are shown. h, BBB leakage, calculated as BBB permeability surface (PS) area product for Texas Red-conjugated dextran, in the cortex of iE3/Cre mice was quantified. i, The frequency and amplitude of arteriolar oscillation in iE3/Cre− and iE3/Cre+ mice were measured. j, Vessel branch densities in the cerebral arterioles of iE3/Cre− and iE3/Cre+ mice were examined. Scale bar, 50 µm. Data expressed as mean ± s.e.m. N.S., not significant, two-tailed Student’s t-test.
Extended Data Fig. 4 Peripheral apoE4 expression is associated with gene expression profiles consistent with reduced vascular functions and compromised energy homeostasis.
Brain cortical tissues from iE4/Cre mice (Cre−, n = 8; Cre+, n = 8) at 12-13 month of age were subjected to RNA-Sequencing. a, Module-genotype correlation. Each rectangle represents a module and selected modules are shown. The number in the front of each module is the correlation coefficient (r) between the module eigengene to genotype; the correlation p-value is in the parentheses. Red represents positive correlation to iE4/Cre+ and blue represents negative correlation to iE4/Cre+. b, Heatmaps of genes within the lightyellow module and selected gene ontologies enriched in the module. c, Interaction of genes involved in the gene ontologies (blue nodes) and hub genes (red nodes) in the lightyellow module. The thickness of the lines represents the strength of gene-gene connection. d, Expression of collagen family members (that is, Col6a3, Col11a2), collagen and calcium binding EGF domain-containing protein 1 (Ccbe1), elastin (Eln), annexin A4 (Anxa4), and genes involved in carbohydrate biosynthetic process as well as the hub genes (Lefty1, Rspo2 and Atp8b1) in the lightyellow module shown in (c) were validated in iE4/Cre mice (Cre−, n = 8; Cre+, n = 8) by real-time PCR analysis. Data expressed as mean ± s.e.m. Ptpn2 (*, P = 0.042); Col6a3 (*, P = 0.036); Col1a2 (*, P = 0.027); Ccbe1 (**, P = 0.006); Eln (*, P = 0.04); Anxa4 (*, P = 0.014); lsyna (*, P = 0.047); Smad6 (*, P = 0.041); Chst12 (*, P = 0.028); Lefty1 (*, P = 0.021); Rspo2 (**, P = 0.006); Atp8b1 (*, P = 0.031), two-tailed Student’s t-test. e, Sunburst plot showing the module hierarchical structure relationship. Each rectangular block denotes a module. A total of 1002 modules were identified in the E4 network, with module size ranging from 10 to 960. The color intensity denotes the FDR adjusted P value significance of GSEA based enrichment for differential expression signals. Functional annotations are highlighted for modules overrepresented with GO/pathway genes (red text for positive GSEA enrichment score with regard to differential expression signal).
Extended Data Fig. 5 Evaluation of glio-vascular-enriched single cell transcriptomics in mice with peripheral expression of apoE isoforms.
Brain cortical tissues from iE3/Cre and iE4/Cre mice (n = 4/genotype) at 12-13 months of age were subjected to vascular and glial cell-enriched single cell RNA-sequencing (scRNA-seq). a, Feature plot of canonical markers defining major cell types. b, Split dot plot depicting marker genes for each cell population in iE3/Cre and iE4/Cre scRNA-seq datasets. Marker genes were identified in an unbiased fashion blind to known cell type markers. The expression level (color intensity) and the percentage of cells in a cluster expressing a given gene (size) are reflected in circles (Cre-, red; Cre+, blue). c, The proportions of cells in each cluster. d, The numbers for each major cell type identified are as follow: Astrocyte (5881), endothelial cell (3970), smooth muscle cell (2495), pericyte (1667), neuron (3014), choroid plexus (2212), microglia (252), and oligodendrocyte (206). The proportions of cell types are shown. e, The number of differentially-expressed genes (DEGs) in glio-vascular unit upon peripheral expression of apoE3 (red) or apoE4 (blue). AC, astrocyte; EC, endothelial cell; FB, fibroblast; SMC, smooth muscle cell; PC, pericyte; MC, myeloid cell; Neu, neuron; CP, choroid plexus.
Extended Data Fig. 6 Brain transcriptional changes in astrocytes influenced by peripheral apoE isoform expression.
a, Violin plots showing the mean and variance differences between iE4/Cre−and iE4/Cre+ astrocytes for genes regulating oxidative stress, lipid metabolism, and hypoxia/stress responses (Selenop, Mfge8, Plpp3, and Vegfa). b, Gene ontology (GO) enrichment analysis for genes upregulated (red) or down-regulated (blue) in astrocytes from iE4/Cre+ mice compared to iE4/Cre− mice. c, Top gene ontology and canonical pathways enriched for DEGs in the astrocytes from iE3/Cre and iE4/Cre mice were identified by gene set enrichment analysis (GSEA). d, Violin plots showing the mean and variance differences between iE4/Cre− and iE4/Cre+ astrocytes for genes regulating astrocyte endfeet (Tmem212, Kcnj10, Sntn, Ankrd66, Stoml3, Pla2g7, Atp2a2). e, Volcano plot depicting up- and down-regulated genes in astrocytic cell populations (AC1, AC2, AC3) in iE3/Cre+ mice compared to iE3/Cre− control mice. Genes significant at the P value ≤ 0.05 and fold change ≥ 1.2 are denoted in red. f, Violin plots showing the mean and variance differences between iE3/Cre− and iE3/Cre+ astrocytes for genes regulating lactate homeostasis (Slc16a1), cellular protection (Psap and Selenow), immune response (Ifi27, Cox5a), and cellular senescence/stress response (Sod1 and Hspa5). g, Gene ontology enrichment analysis for genes up-regulated (orange) or down-regulated (green) in astrocytes from iE3/Cre+ mice compared to iE3/Cre− control mice. Limma’s moderate t-test statistics were used to rank the transcriptome-wide gene-phenotype association.
Extended Data Fig. 7 Reduced vessel-associated astrocytes and microglia in aged mice treated with apoE3 young plasma.
Brain tissues from aged mice treated with Ctrl (PBS/sodium citrate), apoE3, or apoE4 young plasma were subjected to co-immunostaining for GFAP (astrocyte) or Iba1 (for microglia) together with Glut1 (endothelial marker). a, Representative images for the hippocampus of treated experimental mice are shown. Scale bar, 50 µm. b, Quantification of total GFAP and Glut1 (Ctrl: 10 mice; E3: 13 mice; E4: 11 mice) in the hippocampus. **, P < 0.0001. c, Quantification of total Iba1 and Glut1 (Ctrl: 10 mice; E3: 13 mice; E4: 11 mice) in the hippocampus. d, e, Cortical brain tissues from young plasma-treated mice were co-stained for tight junction (TJ) protein ZO1 (green) and an endothelial marker Glut1 (red). Scale bar, 50 µm. e, The total ZO1 and TJ coverage (ZO1 against Glut1) was quantified (Ctrl: 10 mice; E3: 10 mice; E4: 10 mice). *, P = 0.02; **, P = 0.007. f, g, Cortical brain tissues from young plasma-treated mice were co-stained for TJ protein CLDN5 (green) and an endothelial marker Glut1 (red). Scale bar, 50 µm. g, The total CLDN5 and TJ coverage (CLDN5 against Glut1) was quantified (Ctrl: 10 mice; E3: 10 mice; E4: 9 mice). Ctrl vs. E3 (*, P = 0.034); E3 vs. E4 (*, P = 0.044). h, i, Cortical brain tissues from young plasma-treated mice were co-stained for blood protein fibrinogen (green) and an endothelial marker Glut1 (red). Scale bar, 50 µm. i, The total fibrinogen and the fibrinogen/Glut1 signal was quantified (Ctrl: 10 mice; E3: 10 mice; E4: 10 mice). Fibrinogen: Ctrl vs. E3 (*, P = 0.04); E3 vs. E4 (*, P = 0.045). Fibrinogen/Glut1: Ctrl vs. E3 (**, P = 0.001); E3 vs. E4 (*, P = 0.015). j, k, Cortical brain tissues from young plasma-treated mice were co-stained for a pericyte marker CD13 (green) and an endothelial marker Glut1 (red). Scale bar, 50 µm. k, The total CD13 signal and pericyte coverage (CD13 against Glut1) was quantified (Ctrl: 10 mice; E3: 10 mice; E4: 10 mice). b-k, Data represent mean ± s.e.m. N.S., not significant, one-way ANOVA with a Tukey’s post-hoc test.
Extended Data Fig. 8 Liver-specific expression of apoE3 decreases, whereas apoE4 increases Aβ plaque deposition.
a, Brain sections from 9-month-old APP/PS1 mice expressing apoE3 (Cre−, n = 21; Cre+, n = 22) or apoE4 (Cre−, n = 19; Cre+, n = 15) in the liver were immunostained with a pan-Aβ antibody. The Aβ plaque burden in the hippocampus was quantified. Black circle: male; Grey circle: female. E3: *, P = 0.041; E4: **, P = 0.009, two-tailed Student’s t-test. b, Representative images of Aβ staining in the cortex of APP/Alb/iE3 or APP/Alb/iE4 mice (murine Apoe-/- background) are shown. Scale bar, 200 µm. Images from APP/iE mice (murine Apoe+/+ background) were included as visual representation. Note that only diffused plaques were observed in APP/Alb/iE3 or iE4 mice due to the absence of murine apoE in the brain. c, d, TBS- and TBSX-soluble Aβ40 and Aβ42 levels in the cortex of 9-month-old APP/iE3/Cre mice (Cre-, n = 18; Cre+, n = 19) or APP/iE4/Cre mice (Cre-, n = 22; Cre+, n = 25) were examined by specific Aβ ELISA. c, TBS-E3_Aβ40: *, P = 0.036; Aβ42: *, P = 0.045. TBS-E4_Aβ40: **, P = 0.008; Aβ42: **, P = 0.001. d, TBSX-E3_Aβ40: *, P = 0.024; Aβ42: *, P = 0.030. TBSX-E4_Aβ40: **, P = 0.002; Aβ42: **, P = 0.0003. e, f, Brain sections from APP/PS1 mice expressing apoE3 (Cre-, n = 8; Cre+, n = 8) or apoE4 (Cre-, n = 6; Cre+, n = 6) in the liver (murine Apoe-/- background) were labeled for fibrillar Aβ using Thioflavin S (Thio S). Scale bar (upper panels), 1 mm; Scale bar (bottom panels), 100 μm. Images from APP/iE mice (murine Apoe+/+ background) were included for comparison. The amount of fibrillar plaques in the APP/Alb/iE3 or APP/Alb/iE4 mice was minimal due to the absence of murine apoE in the brain. The percentage of area covered by Thio S-positive plaques in the cortex and hippocampus of experimental mice was quantified. g, h, Brain sections from 9-month-old APP/PS1 mice expressing apoE3 (Cre-, n = 27; Cre+, n = 28) or apoE4 (Cre-, n = 19; Cre+, n = 19) in the liver were immunostained with an Iba1 antibody. Scale bar, 100 µm. The immunoreactivity of Iba1 in cortex and hippocampus were quantified. Data expressed as mean ± s.e.m. Cortex: *, P = 0.011; Hippo: *, P = 0.030. N.S., not significant, two-tailed Student’s t-test.
Extended Data Fig. 9 AAV-mediated liver expression of apoE4 enhances amyloid pathology and related toxicity.
a, Schematic illustration of the experimental paradigm. 5xFAD amyloid mice at 1-1.5 month of age were transduced with AAV-Alb-apoE3 or AAV-Alb-apoE4 virus via intravenous injection. b, c, The amyloid deposition in the brain of experimental mice at 4 months of age was examined by immunostaining for Aβ. Scale bar, 1 mm. The amyloid plaque burdens in the cortex and hippocampus (E3, n = 14; E4, n = 16) were quantified. **, P = 0.007. d, e, ApoE levels in the plasma and brain of experimental mice (E3, n = 15; E4, n = 16) were measured by ELISAs. **, P = 0.0098. f, TBSX-soluble and -insoluble (guanidine; GDN) Aβ40 and Aβ42 levels in the cortex of 4-month-old 5xFAD mice transduced with AAV-Alb-apoE3 (n = 15) or AAV-Alb-apoE4 (n = 16) were examined by specific Aβ ELISA. TBSX-Aβ40 (*, P = 0.012); TBSX-Aβ42 (*, P = 0.031). GDN-Aβ40 (*, P = 0.049); GDN-Aβ42 (*, P = 0.033). g, Thio S-positive plaques in the cortex of experimental mice were shown and quantified. Scale bar, 100 µm. h, i, Brain sections from experimental mice (n = 12/genotype) were immunostained with GFAP or Iba1 antibody. Scale bar, 100 µm. The immunoreactivity of GFAP and Iba1 in cortex and hippocampus were quantified. j, Representative images of plaque-associated microglia in mice expressing apoE3 or apoE4 in the liver are shown. Scale bar, 50 μm. The number of Iba1-positive microglia (green) surrounding Aβ plaque (red) between 50-300 µm2 plaque sizes were quantified. Each dot represents the average value from an individual mouse (E3, n = 10; E4, n = 11). *, P = 0.049. k, Co-immunofluorescence staining of LAMP1 (green) and Aβ plaques (red) was used to examine plaque-associated neuritic dystrophy. Scale bar, 50 µm. The LAMP1 immunoreactivity was quantified. *, P = 0.027. l, LAMP1 immunoreactivity was positively correlated with Thio S-positive fibrillar plaques. c-l, Data represent mean ± s.e.m., two-tailed Student’s t-test.
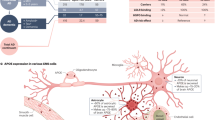
Extended Data Fig. 10 Potential mechanisms by which peripheral apoE4 impacts cerebrovascular integrity, brain function, and AD pathology.
Peripheral apoE4 may modulate plasma factors that promote inflammatory responses and hypoxia/stress in endothelial cells along with cells in the glio-vascular unit51. Tight junction markers (that is, Claudin-5 and ZO1) and astrocyte end-feet marker (that is, AQP4) are down-regulated, whereas the dysregulation of ECM and vessel-associated gliosis are exacerbated in mice expressing apoE4 in the liver. Compromised BBB integrity along with an increase of blood-derived proteins (for example, albumin, IgG, fibrinogen) in the brain, some of which have been shown to increase microglia-mediated oxidative stress and disrupt synaptic function54, may contribute to cognitive deficits in apoE4 mice. Additionally, the impairment of cerebrovascular functions and altered microglial responses may directly or indirectly influence Aβ clearance and Aβ deposition, which together exacerbate amyloid pathology in apoE4 mice. Not depicted here, peripheral apoE3 expression may regulate blood factors that benefit the endothelial integrity and vascular health. For example, Timp3, previously shown to ameliorate the vascular diseases through inhibiting excess matrix metalloproteinase (MMP) activity and inflammation37,49, is elevated in apoE3 mice and increases apoE4 plasma-associated endothelial integrity in the human iPSC-derived cellular model.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8
Supplementary Table 1
Unique proteins quantified in plasma of apoE inducible mice expressing apoE3 or apoE4 in the liver by mass spectrometry.
Supplementary Data 1
Statistical source data for Supplementary Fig. 1.
Supplementary Data 2
Statistical source data for Supplementary Fig. 2.
Supplementary Data 3
Statistical source data for Supplementary Fig. 3.
Supplementary Data 4
Statistical source data for Supplementary Fig. 4.
Supplementary Data 8
Statistical source data for Supplementary Fig. 8.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Fig. 8
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
Liu, CC., Zhao, J., Fu, Y. et al. Peripheral apoE4 enhances Alzheimer’s pathology and impairs cognition by compromising cerebrovascular function. Nat Neurosci 25, 1020–1033 (2022). https://doi.org/10.1038/s41593-022-01127-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01127-0
This article is cited by
-
Association between the circulating very long-chain saturated fatty acid and cognitive function in older adults: findings from the NHANES
BMC Public Health (2024)
-
Deciphering the effect of phytosterols on Alzheimer’s disease and Parkinson’s disease: the mediating role of lipid profiles
Alzheimer's Research & Therapy (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
A year in review: brain barriers and brain fluids research in 2022
Fluids and Barriers of the CNS (2023)
-
Alzheimer’s disease: targeting the peripheral circulation
Molecular Neurodegeneration (2023)