Abstract
Background
Acute kidney injury (AKI) and augmented renal clearance (ARC), both alterations of the glomerular filtration rate (GFR), are prevalent in critically ill children and neonates. AKI and ARC prevalence estimates are based on estimation of GFR (eGFR) using serum creatinine (SCr), which is known to be inaccurate. We aimed to test our hypothesis that AKI prevalence will be higher and ARC prevalence will be lower in critically ill children when using iohexol-based measured GFR (mGFR), rather than using eGFR. Additionally, we aimed to investigate the performance of different SCr-based eGFR methods.
Methods
In this single-center prospective study, critically ill term-born neonates and children were included. mGFR was calculated using a plasma disappearance curve after parenteral administration of iohexol. AKI diagnosis was based on the KDIGO criteria, SCr-based eGFR, and creatinine clearance (CrCL). Differences between eGFR and mGFR were determined using Wilcoxon signed-rank tests and by calculating bias and accuracy (percentage of eGFR values within 30% of mGFR values).
Results
One hundred five children, including 43 neonates, were included. AKI prevalence was higher based on mGFR (48%), than with KDIGO or eGFR (11–40%). ARC prevalence was lower with mGFR (24%) compared to eGFR (38–51%). eGFR equations significantly overestimated mGFR (60–71 versus 41 ml/min/1.73 m2, p < 0.001–0.002). Accuracy was highest with eGFR equations based on age- and sex-dependent equations (up to 59%).
Conclusion
Iohexol-based AKI prevalence was higher and ARC prevalence lower compared to standard SCr-based eGFR methods. Age- and sex-dependent equations for eGFR (eGFR-Smeets for neonates and eGFR-Pierce for children) best approached measured GFR and should preferably be used to optimize diagnosis of AKI and ARC in this population.
Graphical Abstract
A higher resolution version of the Graphical abstract is available as Supplementary information

Similar content being viewed by others
Background
Critically ill children and neonates are at risk for acute kidney injury (AKI), resulting in a sudden derangement of glomerular filtration rate (GFR). This is an independent risk factor for prolonged mechanical ventilation, extended stay in the intensive care unit (ICU), and higher mortality [1]. Augmented renal clearance (ARC), which is enhanced kidney perfusion and glomerular hyperfiltration, is also prevalent in critically ill children [2]. As altered GFR affects fluid and electrolyte management, and requires dose-adaptation drugs cleared by the kidneys, accurate and timely diagnosis of both AKI and ARC is crucial.
The diagnosis of AKI and ARC in clinical care is mostly based on imperfect parameters including serum creatinine (SCr) levels and urine output (UO) [1]. Yet, SCr has several drawbacks and, in addition to glomerular filtration, is also cleared by tubular secretion. Especially in neonates, obtaining accurate GFR estimates is challenging as SCr values reflect maternal creatinine levels and GFR increases in the first days of life [3, 4]. In addition, other endogenous markers for GFR exist, but are rarely used in daily clinical care.
As an alternative to estimated GFR (eGFR) using endogenous biomarkers, GFR can be measured using exogenous substances, such as inulin, radio-isotopes, and iohexol. These markers are inert, non-protein bound, exclusively cleared by glomerular filtration, and free of any tubular handling. Hence, their use to measure GFR (mGFR) results in more accurate determination of GFR than SCr-based estimations and is considered the gold standard for GFR determination [5, 6]. More specifically, iohexol clearances were validated against urinary inulin clearance, and very good agreement was demonstrated in both the adult [7] and pediatric population [8]. To overcome some of the limitations of SCr, and to prevent the need to administer an exogenous compound, creatinine clearance (CrCL), based on Scr urinary creatinine levels and UO, may also be used as it may approach mGFR [9].
In relatively small cohorts of adult ICU patients (18–34 patients), iohexol-based mGFR was compared to SCr-based eGFR [10, 11] or CrCL [12]. In these cohorts, SCr-based eGFR equations showed high inaccuracy when compared to iohexol-based mGFR in critically ill adults [13], resulting in misdiagnosis of AKI and ARC. Because developmental changes in kidney function occur including maturation of GFR and tubular excretion [4], adult findings cannot easily be extrapolated to children. Until now, no mGFR studies were conducted in critically ill children. We hypothesize that SCr-based GFR estimations are inaccurate, leading to underdiagnosis of AKI and overdiagnosis of ARC. Therefore, we aimed to measure GFR using iohexol in critically ill children and term-born neonates. Our primary objective was to compare the prevalence of AKI and ARC between iohexol-based mGFR and SCr, UO, eGFR, and CrCL-based diagnosis. As a secondary objective, we investigated the performance of commonly used SCr-based eGFR methods compared to iohexol-based mGFR as gold standard.
Methods
Study design
The methods of our single-center prospective study are described according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [14]. Details of the Iohexol for Measuring Renal Function (HERO) study were registered on ClinicalTrials.gov (registration number NCT03946345) before start of the study. The HERO study protocol was approved by the Medical Ethics Review Board (CMO Arnhem-Nijmegen, NL68547.091.18, 2018–5025).
Setting
The study was performed at the Radboud University Medical Center, Nijmegen, Netherlands, a tertiary teaching hospital providing intensive care to children and neonates. Patients were recruited from the PICU (approximately 570 admissions yearly), and the NICU (200 term newborn admissions yearly), and data were collected between May 2019 and July 2021.
Patients
Patients were eligible for inclusion if they were below 18 years of age, term-born (≥ 37 weeks of gestation, if < 1 year of age), had a bodyweight of more than 2500 g, and at least one failing organ as defined by a Pediatric Logistic Organ Dysfunction II (PELOD-II) score of 1 or higher (range 0–33) [15]. They also needed to have an indwelling central venous or arterial line already in place for clinical purposes. Exclusion criteria were a known medical history of allergic reaction to injection of iodinated contrast material, receiving kidney replacement therapy or extra corporeal membrane oxygenation (ECMO), and language or cognitive inability of parents/caregivers to understand written and/or oral information. Informed consent needed to be provided by parents or other legal representatives if the child was below 16 years of age. Consent of the child was needed if aged above 12 years of age and medical and cognitive state permitted.
Diagnostic test
After inclusion, iohexol (Omnipaque® 300 mg/mL, GE Healthcare, Chicago, Illinois, USA) was administered as a single bolus dose adapted to bodyweight as follows: < 10 kg, 1 mL; 10–20 kg, 2 mL; 20–30 kg, 3 mL; 30–40 kg, 4 mL; and ≥ 40 kg, 5 mL [16]. To determine mGFR, blood samples were drawn for analysis of iohexol concentrations at 2, 5, and 7 h after administration. Two-point blood sampling at 2 and 5 h after administration is a validated method for mGFR determination in children [16]. To enhance accuracy as low GFR values were expected, we added another sampling point at 7 h after administration for neonates with a bodyweight of at least 3.5 kg and children older than 28 days of age. SCr levels were determined 2 h after iohexol infusion for eGFR determination to reflect the clinical situation in which SCr is measured at point of care and to correspond to the first blood withdrawal point needed for mGFR, preventing an extra blood withdrawal. Urine was collected for 2 h between 4 and 6 h after infusion of iohexol for urine creatinine levels, to calculate CrCL and corresponding to the second blood withdrawal needed for mGFR at 5 h. We opted to collect urine around the second blood withdrawal, to ensure this corresponds to the elimination phase of iohexol.
Analytical procedures
SCr was assessed by an enzymatic assay (Creatinine Plus, Roche Diagnostics, Meylan, France). Iohexol plasma concentrations were determined at the Leiden University Medical Centre, Leiden, Netherlands, using a validated high-performance liquid chromatography diode array detection assay [17]. The assay was validated according to the European Medicines Agency bioanalytical method validation guidelines [18].
Variables
Data were retrieved from the electronic health record and involved demographic data, laboratory results, and observational data of physiological parameters. Demographic data included postnatal age, gestational age, gender, height, and weight. Co-existing conditions and comedication, i.e., vaso-active medication and/or nephrotoxic medication (Supplementary Table S1) and disease severity scores (Pediatric Risk of Mortality III (PRISM-III) [19], Pediatric Index of Mortality 2 (PIM2) [20], and Score for Neonatal Acute Physiology, second version (SNAP-II) [21]) (for neonates only), were collected at the time of inclusion. In addition, the duration of ICU admission was recorded. The amount of urine excreted per hour, corrected for bodyweight (UO), was registered at 8-h intervals.
Calculation of GFR
Iohexol-based mGFR and eGFR were determined in each patient at a standardized timepoint early at admission, regardless of clinical status or AKI diagnosis, in order to reflect GFR for the entire critically ill population.
Iohexol-based mGFR
Calculations to determine mGFR in children using iohexol were previously published [16] and are listed in the “Supplementary information” (Equations S1-S4). mGFR based on iohexol plasma clearance was calculated based on the ratio between the administered iohexol dose and the area under the plasma concentration time curve. A slope-intercept method, using the Jødal and Brøchner–Mortensen (JBM) formula with early normalization to 1.73 m2 body surface area (BSA), was employed as this method was previously validated in children with CKD [16, 22]. The Haycock formula was used to calculate BSA [23, 24].
eGFR
eGFR based on serum creatinine was estimated using one formula including different fixed and age-specific coefficients (k):
First, the frequently used Schwartz equation was used with fixed coefficients reported for children below (k = 44) and above 1 year of age (k = 41.3) (eGFR-bedside) [25, 26]. For neonates, a coefficient of 31.0 was also tested (eGFR-Smeets) as high accuracy was found using an individual participant data meta-analysis reporting mGFR reference values for healthy term-born neonates (data accepted for publication in Journal of American Society of Nephrology). Additionally, different age- and sex-specific k values as reported by Pierce et al., ranging from 33.1 for females of 1 year of age to 48.6 for males of 17 years of age, were used (eGFR-Pierce) [27]. For this equation, the k value as reported for 1-year-olds was used for patients below 1 year of age as no specific coefficients for younger children were reported.
CrCL was calculated using 2-h urine collection intervals [28]:
Definition of AKI and ARC
The prevalence and severity of AKI were determined using mGFR and seven other diagnostic criteria. First, AKI was diagnosed using SCr, UO, and a combination of SCr and UO with the Kidney Disease Improving Global Outcomes (KDIGO) criteria. The KDIGO categories risk (stage 1), injury (stage 2), and failure (stage 3) were defined using median age-specific reference values of enzymatic SCr for neonates [29] and children from 28 days of age [30] to circumvent the lack of baseline SCr values due to unplanned admissions. AKI categories were defined as > 150%, > 200%, and > 300% of median age-specific reference values for SCr, as this approach was previously described by Zwiers et al. to diagnose AKI in critically ill infants [31] Next, in order to enable comparison between mGFR- and eGFR-based diagnosis, eGFR- and mGFR-based prevalence and severity of AKI and ARC was defined based on mGFR and eGFR methods. Three categories of severity were defined based on the mean age-specific reference values for GFR [31]. Again, separate reference values for neonates [32] and children from 1 month of age onward were applied [33]. AKI categories were defined as follows: stage 1, meanage – 1 SDage > GFR ≥ meanage – 1.5 SDage; stage 2, meanage – 1.5 SDage > GFR ≥ meanage – 2 SDage; and stage 3, GFR < meanage – 2 SDage. Because no universal ARC diagnostic criteria exist, they were defined using the opposite AKI criteria by applying + 1, + 1.5, and + 2 SD as cut-off values for the different stages. The prevalence of AKI was reported including all stages as well as dichotomized at stage 2 (only including stage 2 and 3).
Statistical analysis
Sample size
Our sample size was based on the primary study aim. We implemented an expected true proportion of AKI (p) of 50% in our calculations, as the SCr-based prevalence in critically ill neonates was 35% [31] and we expected mGFR-based prevalence to be higher. Also, we applied a desired precision (d) of 0.1. By using a standard 95% confidence interval (CI), we applied a Z-score of 1.96 resulting in a sample size (n) of 98, calculated by n = (Z×p)/d. Accounting for drop-outs, 105 patients were included.
Demographics
All demographic data were analyzed for the entire study cohort as well as separately for neonates (≤ 28 days of postnatal age) and children (> 28 days of postnatal age). For continuous variables, data were expressed as median values with interquartile ranges (IQR) or ranges, whereas for categorical variables, numbers and percentages were used.
Primary objective: AKI and ARC prevalence and severity
The number of missing values per mGFR and eGFR method was displayed, and prevalence and performance were calculated using available data only. In case only two iohexol concentrations were available, mGFR was calculated using two points. Missing values were not replaced. AKI and ARC prevalences were calculated, and differences in prevalence and severity were compared using a McNemar and McNemar-Bowker Test of Symmetry.
Secondary objective: performance of eGFR equations
The comparison of GFR methods for each patient was based on one standardized point in time early at admission, regardless of clinical status or AKI diagnosis. To assess the agreement between several eGFR equations with iohexol-based mGFR, bias and accuracy were calculated. GFR data were analyzed by calculating the difference between eGFR and mGFR per patient and determining the median of this difference (bias). Comparison of eGFR and mGFR values on a group level was performed using the Wilcoxon signed-rank test for paired data. Accuracy was calculated as the percentage of patients having a similar eGFR when compared to mGFR (≤ 30% difference) [13].
Data were analyzed by using SPSS version 25.0.0.1 for Windows (SPSS, Chicago, IL, USA).
Results
Patients
From May 2019 until June 2021, 611 PICU patients and 225 term-born NICU patients were admitted and screened for eligibility (Fig. 1). Of these 836 patients, 192 were eligible. Forty-four patients were eligible but not approached for informed consent due to expected death within 1 day, transfer to regular ward on the same day or based on advice of the treating physician. In total, 108 out of 148 approached patients/parents/legal representatives provided written informed consent. Of these patients, two patients lost their indwelling catheter and for one patient, consent was withdrawn. As no iohexol concentration–time profile could be determined in these patients, they were excluded from further analysis. Of all patients, one (AKI stage 3, according to all different methods) required kidney replacement therapy after study completion, and ten passed away. The patient characteristics are presented for neonates (n = 43) and children (n = 62) (Table 1). At inclusion, median age was 6.1 years (range 0–17 years) for children and 0 days (range 0–27 days) for neonates. Iohexol was administered after a median duration of 26 h (IQR 16–52) after admission. For 101 patients, mGFR was available. One hundred four patients had SCr values and from 96 patients urinary output data were available. Urinary CrCL values were available for 81 patients.
Patient inclusions. Neonates are ≤ 28 days of age, whereas children are > 28 days of age. Abbreviations: PELOD-II-score, Pediatric Logistic Organ Dysfunction version 2 score; HERO, Iohexol for Measuring Renal Function; ECMO, extra-corporeal membrane oxygenation; mGFR, measured glomerular filtration rate
AKI prevalence
Iohexol-based (mGFR) AKI prevalence was 48% compared to 11–40% for KDIGO- or eGFR-based diagnosis (Fig. 2). For neonates, this was 56% for mGFR and 8–53% for KDIGO- or eGFR-based diagnosis. Also, in children, a similar pattern was observed with 43% mGFR-based prevalence compared to 14–37% for other methods. Staging of AKI differed significantly between the methods (McNemar-Bowker test, p = < 0.001–0.037), except between mGFR and eGFR-Pierce for both neonates and children (p = 0.127), and, in neonates, between mGFR and eGFR-Smeets (p = 0.236). When dichotomized at stage 2, the observed trend was similar. mGFR-based AKI prevalence was 40% compared to 6–35% for KDIGO- or eGFR-based diagnosis. For neonates, this was 46% for mGFR-based diagnosis versus 3–51% for KDIGO- or eGFR-based diagnosis. In children, AKI prevalence was 35% when diagnosis was based on mGFR, compared to 9–29% for other methods.
Prevalence of AKI using mGFR and different eGFR based methods. Statistical significance differences (p < 0.05) based on McNemar Bowker test for symmetry between eGFR and mGFR defined AKI are indicated by *. Abbreviations: SCr, serum creatinine; UO, urine output; mGFR, measured glomerular filtration rate; eGFR, estimated glomerular filtration rate; CrCL, creatinine clearance; AKI, acute kidney injury
ARC prevalence
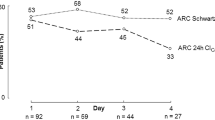
ARC prevalence was 24% when based on mGFR, as opposed to 38–51% using eGFR methods (Fig. 3). In neonates, this was 29% for mGFR and 32–65% for eGFR, whereas in children, this was 21% for mGFR and 35–52% for eGFR. Again, staging of ARC differed significantly between mGFR and eGFR (McNemar Bowker test, p = < 0.001–0.018).
Prevalence of ARC using mGFR and different eGFR-based methods. Statistically significant differences (p < 0.05) based on McNemar Bowker test for symmetry between eGFR and mGFR defined ARC are indicated by *. Abbreviations: mGFR, measured glomerular filtration rate; eGFR, estimated glomerular filtration rate; CrCL, creatinine clearance; ARC, augmented renal clearance
Performance of eGFR equations
Median mGFR was 40.6 (IQR 26.4–92.9) ml/min/1.73 m2. In children, median mGFR was 80.7 (IQR 42.3–114.7) versus 29.2 (IQR 22.3–35.0) ml/min/1.73 m2 in neonates (Table 2). For all methods, with the exception of eGFR-Pierce in neonates and eGFR-Smeets in neonates, mGFR was significantly lower than eGFR (p = < 0.001–0.016). Median difference between eGFR and mGFR ranged from 0.0 (IQR − 6.4 to 5.2) ml/min/1.73 m2 for eGFR-Smeets in neonates up to 28.7 (IQR 5.6–88.1) ml/min/1.73 m2 for CrCL in children. Accuracy varied accordingly between 19.4 and 74.4%. Analysis was repeated for patients without AKI only, which yielded similar results (Supplementary Table S2).
Discussion
Key results
We demonstrated that the prevalence of AKI and ARC between iohexol-based mGFR and eGFR differed, as hypothesized. AKI prevalence was higher (49%) when using mGFR compared to KDIGO- or eGFR-based diagnosis (11–40%). Similarly, mGFR-based ARC prevalence was lower (24%) compared to diagnosis based on various eGFR methods (38–51%). Also, all clinically used eGFR methods presented significant biases and poor accuracy when compared to mGFR values, thereby significantly overestimating GFR (between 2.0 and 28.7 ml/min/1.73 m2). Of all equations, in children, the eGFR-Pierce and in neonates, the eGFR-Smeets demonstrated the highest accuracy and lowest bias and could therefore be used in clinical care to improve GFR estimations in critically ill children and neonates.
Interpretation of AKI and ARC prevalence
Our data are innovative; as to the best of our knowledge, this is the first study in critically ill children and/or term-born neonates, and our cohort is by far the largest of critically ill patients, as adult studies included up to 66 patients. Additionally, both AKI and ARC were assessed using mGFR in the same ICU population. Our cohort is representative of other pediatric ICU cohorts, as our SCr and/or UO-based AKI prevalence (23–29%) is similar to previously published AKI prevalences in critically ill children (27%) and in infants (< 1 year of age) (35%) [1, 31]. The lack of pediatric mGFR studies prevents comparison to other pediatric mGFR data. In the large AWAKEN cohort, in nearly one in five pediatric AKI patients, SCr was not increased, but diagnosis was based on reduced UO [34]. Also, in our cohort, in 24% of mGFR-based AKI patients SCr levels were not increased. Yet, by including UO as diagnostic criterion for AKI, only two additional patients were diagnosed compared to SCr-based criteria in our cohort (data not shown).
ARC prevalence in critically ill children has only been reported using vancomycin clearance as a surrogate of mGFR [35]. By defining ARC as vancomycin clearance of ≥ 130 ml/min/1.73 m2 regardless of age, 12% of 250 critically ill children were diagnosed with ARC, compared to 24% in our cohort. A limitation of this study was the use of an age-independent cut-off for clearance, ignoring age-related GFR changes. This could have led to an underestimation of ARC prevalence. When AKI and ARC patients are not classified as such, drug dosing might be suboptimal leading to therapy failure or toxicity. This poses patients at risk for adverse outcomes [2, 36]. In our cohort, neither ventilator-free days nor duration of stay differed between mGFR-based AKI patients who would have been missed using KDIGO-based diagnosis only, compared to both KDIGO- and mGFR-based AKI diagnosis (data not shown). This could be due to the relatively short follow-up and limited sample size of our cohort. Whether toxicity or therapy failure could be diminished by using mGFR-based diagnosis remains to be studied in a larger cohort.
Interpretation of performance of eGFR methods
Our results illustrate the difference in performance of different eGFR methods and highlight the importance of using age-specific SCr-based eGFR equations (i.e., eGFR-Smeets for neonates and eGFR-Pierce for children) as these give the best approximation of GFR. To date, no mGFR validation studies in critically ill children have been performed, preventing comparison of our results to other pediatric cohorts. However, seven studies in critically ill adults (n = 18–66) investigated the performance of several eGFR equations. Similar to our results, multiple SCr-based eGFR equations displayed high biases and low accuracy (23–60%, dependent on equation used) when compared with mGFR values [11, 13, 28, 37,38,39,40].
The inaccuracy of SCr-based eGFR equations can be understood by understanding the pharmacokinetic properties of this marker, next to the other drawbacks of SCr as mentioned in the introduction. Compared to iohexol, the volume of distribution of creatinine is larger as it distributes into the total body water (TBW), whereas iohexol is only distributed in the extracellular volume (ECV) [41]. Correspondingly, because the ECV comprises around one-third of the TBW, creatinine half-life is around three times higher than iohexol half-life [42]. eGFR based on creatinine is therefore reflecting GFR over a longer (preceding) period. Consequently, iohexol-based mGFR is able to detect changes in GFR earlier than SCr-based eGFR. Even though the performance of all SCr-based eGFR equations is limited in critically ill patients, there are significant differences in performance between the different eGFR equations. Attention should therefore be paid to use and implement the best equation for each patient population with mGFR. Using the appropriate formula (i.e., eGFR-Smeets for neonates and eGFR-Pierce for children) could improve GFR estimations in clinical care.
Because of the limitations of SCr, cystatin C (cysC) has been proposed as an alternative marker of GFR. CysC has consistent stable plasma levels from 1 year of age, and its levels are independent of muscle mass [43]. Also, cysC is not secreted by the tubules, and distributed in the ECV only, explaining the shorter half-life compared to creatinine. Therefore, the value of cysC as a marker for GFR in the neonatal and pediatric population has been investigated by many and is considered promising. However, cysC levels are significantly affected by thyroid disorders [44] and corticosteroids [45]. Whether cysC-based eGFR equations are of value in critically ill neonates and children remains to be validated.
Limitations
Our study has some limitations. First, we considered three-point iohexol clearance as the gold standard for mGFR as opposed to a rich sampling schedule, as optimal sampling schemes with more than three sampling points only marginally increased accuracy and precision [17, 46, 47]. We therefore believe this did not affect our conclusions. Additionally, by using an extra sampling point at 7 h, we optimized accuracy to account for low GFR. Because critical illness is highly dynamic and GFR shows an evolution over time, using single-bolus iohexol plasma clearance will only allow for the calculation of mean GFR over the sampling period, in our case 7 h. Consequently, GFR results will become available after this period of time. When using continuous (low-dose) iohexol infusion, this problem might be circumvented as steady state is immediately obtained after a loading dose is administered [48], and clearance can be calculated at point of care. However, administering the very small neonatal doses in a continuous manner is impractical with the current infusion pumps, and occupying a lumen of an IV line for the entire day is undesirable. Hence, we used single-bolus infusion of iohexol in our cohort. Next to the “real” differences between SCr-based eGFR and mGFR discussed above, these limitations could also have contributed to the observed differences between eGFR and mGFR.
Future perspectives
Routine measurements of iohexol-based GFR are increasingly used in standard of care, especially in (pediatric) CKD patients, in the kidney transplant setting, and in oncology [16, 17, 49]. The use of iohexol as a marker for GFR is safe [7, 50] and iohexol has a low interaction potential [49]. This enables its use in the intensive care setting where patients receive multiple therapies. Also, the calculation needed to calculate GFR based on measured concentrations is easy to implement in clinical support systems as demonstrated by Zwart et al. [17], making mGFR results directly visible for treating physicians. Understandably, all iohexol adult studies advocated the use of routine iohexol measurements in the intensive care setting [11, 13, 28, 37,38,39,40].
Contrary to these adult studies, we believe, at this point in time, that the added value of increased accuracy of GFR determination using iohexol plasma clearance in the neonatal and pediatric intensive care setting will not outweigh the disadvantages of the cumbersome procedure. In addition, the lack of extensive availability to analyze iohexol plasma concentrations at the point of care and to perform pharmacokinetic analysis of data 24/7 in most centers will hamper its direct implementation. However, for certain patients in which the use of SCr as a marker for GFR is severely hampered (e.g., low muscle mass), it might be worthwhile to determine mGFR to link SCr levels to GFR. Also, future perspectives regarding mGFR determination are promising. To circumvent the impracticality of GFR measurements, the plasma clearance of iohexol can be calculated using modelling and simulation approaches based on few blood withdrawals within 4 h after administration. This method of GFR determination has already been implemented in adult kidney transplantation care [17] and would be of great value in the intensive care setting. Furthermore, novel technologies provide opportunities for the continuous monitoring of GFR by transdermal measurement of a fluorescent GFR marker [51, 52]. Without losing accuracy and precision, these methods could facilitate the use of rapid and reliable determination of GFR at the bedside without the need for indwelling catheters.
Until iohexol-based GFR measurements using sparse sampling schedules are available for critically ill children and neonates at point of care, including the best eGFR equation in electronic decision support systems that present eGFR when creatinine values and height are available, is of great importance. According to our findings, we propose the eGFR-Pierce for children and eGFR-Smeets for neonates. As GFR-adjusted dosing has been shown to optimize drug target concentrations for drugs cleared by the kidneys [53], using these eGFR equations could prevent over- or under-dosing of drugs cleared by the kidney and might optimize fluid and electrolyte management in AKI or ARC patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKI:
-
Acute kidney injury
- ARC:
-
Augmented renal clearance
- BSA:
-
Body surface area
- BUN:
-
Blood urea nitrogen
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CL:
-
Clearance
- CrCL:
-
Creatinine clearance
- cysC:
-
Cystatin C
- ECMO:
-
Extracorporeal membrane oxygenation
- eGFR:
-
Estimated glomerular filtration rate
- GFR:
-
Glomerular filtration rate
- ICU:
-
Intensive care unit
- JBM:
-
Jødal and Brøchner–Mortensen
- k:
-
Coefficient
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- mGFR:
-
Measured glomerular filtration rate
- NICU:
-
Neonatal intensive care unit
- PELOD-II:
-
Pediatric Logistic Organ Dysfunction, second version
- PICU:
-
Pediatric intensive care unit
- PIM2:
-
Pediatric Index of Mortality, second version
- PRISM-III:
-
Pediatric Risk of Mortality, third version
- SCr:
-
Serum creatinine
- SNAP-II:
-
Score for Neonatal Acute Physiology, second version
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- UO:
-
Urine output
- Vd:
-
Volume of distribution
References
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20
Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA (2020) Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol 35:25–39
Schwartz GJ, Feld LG, Langford DJ (1984) A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104:849–854
Chen N, Aleksa K, Woodland C, Rieder M, Koren G (2006) Ontogeny of drug elimination by the human kidney. Pediatr Nephrol 21:160–168
Brown SC, O’Reilly PH (1991) Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J Urol 146:675–679
Delanaye P, Ebert N, Melsom T, Gaspari F, Mariat C, Cavalier E, Bjork J, Christensson A, Nyman U, Porrini E, Remuzzi G, Ruggenenti P, Schaeffner E, Soveri I, Sterner G, Eriksen BO, Back SE (2016) Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin Kidney J 9:682–699
Delanaye P, Melsom T, Ebert N, Back SE, Mariat C, Cavalier E, Bjork J, Christensson A, Nyman U, Porrini E, Remuzzi G, Ruggenenti P, Schaeffner E, Soveri I, Sterner G, Eriksen BO, Gaspari F (2016) Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin Kidney J 9:700–704
Berg UB, Back R, Celsi G, Halling SE, Homberg I, Krmar RT, Monemi KA, Oborn H, Herthelius M (2011) Comparison of plasma clearance of iohexol and urinary clearance of inulin for measurement of GFR in children. Am J Kidney Dis 57:55–61
Arant BS Jr, Edelmann CM Jr, Spitzer A (1972) The congruence of creatinine and inulin clearances in children: use of the Technicon AutoAnalyzer. J Pediatr 81:559–561
Dixon JJ, Lane K, Dalton RN, Turner C, MacPhee IAM, Chis Ster I, Philips BJ (2018) Continuous infusion of low-dose iohexol measures changing glomerular filtration rate in critically ill patients. Crit Care Med 46:e190–e197
Salmon-Gandonniere C, Benz-de Bretagne I, Mercier E, Joret A, Halimi JM, Ehrmann S, Barin-Le Guellec C (2016) Iohexol clearance in unstable critically ill patients: a tool to assess glomerular filtration rate. Clin Chem Lab Med 54:1777–1786
Collet M, Hijazi D, Sevrain P, Barthélémy R, Labeyrie MA, Prié D, Tabibzadeh N, Mebazaa A, Chousterman BG (2021) Evaluation of glomerular filtration rate using iohexol plasma clearance in critically ill patients with augmented renal creatinine clearance: A single-centre retrospective study. Eur J Anaesthesiol 38:652–658
Sangla F, Marti PE, Verissimo T, Pugin J, de Seigneux S, Legouis D (2020) Measured and estimated glomerular filtration rate in the ICU: a prospective study. Crit Care Med 48:e1232–e1241
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F (2013) PELOD-2: an update of the PEdiatric logistic organ dysfunction score. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf41:1761-1773. Accessed 1 Sept 2018
Tondel C, Bolann B, Salvador CL, Brackman D, Bjerre A, Svarstad E, Brun A (2017) Iohexol plasma clearance in children: validation of multiple formulas and two-point sampling times. Pediatr Nephrol 32:311–320
Zwart TC, de Vries APJ, Engbers AGJ, Dam RE, van der Boog PJM, Swen JJ, Keizer RJ, Dalton RN, Guchelaar HJ, de Fijter JW, Moes D (2021) Model-based estimation of iohexol plasma clearance for pragmatic renal function determination in the renal transplantation setting. Clin Pharmacokinet 60:1201–1215
Committee for Medicinal Products for Human Use (CHMP). European Medicines Agency (2011) Guideline on bioanalytical method validation. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf. Accessed 1 Oct 2018
Pollack MM, Patel KM, Ruttimann UE (1996) PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 24:743–752
Slater A, Shann F, Pearson G (2003) PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 29:278–285
Richardson DK, Corcoran JD, Escobar GJ, Lee SK (2001) SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr 138:92–100
Jodal L, Brochner-Mortensen J (2009) Reassessment of a classical single injection 51Cr-EDTA clearance method for determination of renal function in children and adults. Part I: Analytically correct relationship between total and one-pool clearance. Scand J Clin Lab Invest 69:305–313
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93:62–66
van der Sijs H, Guchelaar HJ (2002) Formulas for calculating body surface area. Ann Pharmacother 36:345–346
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34:571–590
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ (2020) Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int 99:948–956
Bragadottir G, Redfors B, Ricksten SE (2013) Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury–true GFR versus urinary creatinine clearance and estimating equations. Crit Care 17:R108
Boer DP, de Rijke YB, Hop WC, Cransberg K, Dorresteijn EM (2010) Reference values for serum creatinine in children younger than 1 year of age. Pediatr Nephrol 25:2107–2113
Pottel H, Vrydags N, Mahieu B, Vandewynckele E, Croes K, Martens F (2008) Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta 396:49–55
Zwiers AJ, de Wildt SN, van Rosmalen J, de Rijke YB, Buijs EA, Tibboel D, Cransberg K (2015) Urinary neutrophil gelatinase-associated lipocalin identifies critically ill young children with acute kidney injury following intensive care admission: a prospective cohort study. Crit Care 19:181
Smeets N, IntHout J, van der Burgh M, Schwartz G, Schreuder M, de Wildt S (2022) Maturation of Glomerular Filtration Rate in Term-Born Neonates: An Individual Participant Data Meta-Analysis. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2021101326
Piepsz A, Tondeur M, Ham H (2006) Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging 33:1477–1482
Kaddourah A, Basu RK, Goldstein SL, Sutherland SM (2019) Oliguria and acute kidney injury in critically ill children: implications for diagnosis and outcomes. Pediatr Crit Care Med 20:332–339
Avedissian SN, Bradley E, Zhang D, Bradley JS, Nazer LH, Tran TM, Nguyen A, Le J (2017) Augmented renal clearance using population-based pharmacokinetic modeling in critically ill pediatric patients. Pediatr Crit Care Med 18:e388–e394
Bellos I, Daskalakis G, Pergialiotis V (2020) Relationship of vancomycin trough levels with acute kidney injury risk: an exposure-toxicity meta-analysis. J Antimicrob Chemother 75:2725–2734
Carlier M, Dumoulin A, Janssen A, Picavet S, Vanthuyne S, Van Eynde R, Vanholder R, Delanghe J, De Schoenmakere G, De Waele JJ, Hoste EA (2015) Comparison of different equations to assess glomerular filtration in critically ill patients. Intensive Care Med 41:427–435
Delanaye P, Cavalier E, Morel J, Mehdi M, Maillard N, Claisse G, Lambermont B, Dubois BE, Damas P, Krzesinski JM, Lautrette A, Mariat C (2014) Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol 15:9
Brondén B, Eyjolfsson A, Blomquist S, Dardashti A, Ederoth P, Bjursten H (2011) Evaluation of cystatin C with iohexol clearance in cardiac surgery. Acta Anaesthesiol Scand 55:196–202
Erley CM, Bader BD, Berger ED, Vochazer A, Jorzik JJ, Dietz K, Risler T (2001) Plasma clearance of iodine contrast media as a measure of glomerular filtration rate in critically ill patients. Crit Care Med 29:1544–1550
Pickering JW, Ralib AM, Endre ZH (2013) Combining creatinine and volume kinetics identifies missed cases of acute kidney injury following cardiac arrest. Crit Care 17:R7
Chiou WL, Hsu FH (1975) Pharmacokinetics of creatinine in man and its implications in the monitoring of renal function and in dosage regimen modifications in patients with renal insufficiency. J Clin Pharmacol 15:427–434
Allegaert K, Mekahli D, van den Anker J (2015) Cystatin C in newborns: a promising renal biomarker in search for standardization and validation. J Matern Fetal Neonatal Med 28:1833–1838
Pöge U, Gerhardt T, Bökenkamp A, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP (2004) Time course of low molecular weight proteins in the early kidney transplantation period–influence of corticosteroids. Nephrol Dial Transplant 19:2858–2863
Jayagopal V, Keevil BG, Atkin SL, Jennings PE, Kilpatrick ES (2003) Paradoxical changes in cystatin C and serum creatinine in patients with hypo- and hyperthyroidism. Clin Chem 49:680–681
Åsberg A, Bjerre A, Almaas R, Luis-Lima S, Robertsen I, Salvador CL, Porrini E, Schwartz GJ, Hartmann A, Bergan S (2020) Measured GFR by utilizing population pharmacokinetic methods to determine iohexol clearance. Kidney Int Rep 5:189–198
Delanaye P, Flamant M, Dubourg L, Vidal-Petiot E, Lemoine S, Cavalier E, Schaeffner E, Ebert N, Pottel H (2018) Single- versus multiple-sample method to measure glomerular filtration rate. Nephrol Dial Transplant 33:1778–1785
Dixon JJ, Lane K, Dalton RN, Turner C, Grounds RM, MacPhee IA, Philips BJ (2015) Validation of a continuous infusion of low dose Iohexol to measure glomerular filtration rate: randomised clinical trial. J Transl Med 13:58
Joshi A, Guo J, Holleran JL, Kiesel B, Taylor S, Christner S, Parise RA, Miller BM, Ivy SP, Chu E, Venkataramanan R, Beumer JH (2020) Evaluation of the pharmacokinetic drug-drug interaction potential of iohexol, a renal filtration marker. Cancer Chemother Pharmacol 86:535–545
Gaspari F, Thakar S, Carrara F, Perna A, Trillini M, Aparicio MC, Diadei O, Ferrari S, Cannata A, Stucchi N, Ruggenenti P, Remuzzi G, Perico N (2018) Safety of iohexol administration to measure glomerular filtration rate in different patient populations: a 25-year experience. Nephron 140:1–8
Solomon R, Goldstein S (2017) Real-time measurement of glomerular filtration rate. Curr Opin Crit Care 23:470–474
Rizk DV, Meier D, Sandoval RM, Chacana T, Reilly ES, Seegmiller JC, DeNoia E, Strickland JS, Muldoon J, Molitoris BA (2018) A novel method for rapid bedside measurement of GFR. J Am Soc Nephrol 29:1609–1613
Smits A, Annaert P, Allegaert K (2013) Drug disposition and clinical practice in neonates: cross talk between developmental physiology and pharmacology. Int J Pharm 452:8–13
Acknowledgements
We would like to thank Lieke Raaijmakers, Jara Stevens, and Meris Gutiç for their help in collecting the samples. We thank Rogier Donders for his statistical support.
Funding
The research was funded by the Radboud University Medical Center.
Author information
Authors and Affiliations
Contributions
NS contributed to the study conception or design, and the acquisition, analysis, and interpretation of data. CT, DM, RH, AH, MS, and SW contributed to the study conception or design and the interpretation of data. MS and SW supervised the study. ET, KV, MB, DL, and ET contributed to the analysis and/or interpretation of data. All authors revised the work critically for important intellectual content and provided final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Iohexol for Measuring Renal Function (HERO) study was conducted in accordance with the principles of Good Clinical Practice. Details of this study were registered in advance on ClinicalTrials.gov on May 10, 2019 (registration number NCT03946345). The HERO study protocol was approved by the local medical ethics review board (CMO Arnhem-Nijmegen, NL68547.091.18, 2018–5025).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1
(DOCX 18 kb)
Graphical Abstract
(PPTX 169 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smeets, N.J.L., Teunissen, E.M.M., van der Velden, K. et al. Glomerular filtration rate in critically ill neonates and children: creatinine-based estimations versus iohexol-based measurements. Pediatr Nephrol 38, 1087–1097 (2023). https://doi.org/10.1007/s00467-022-05651-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05651-w