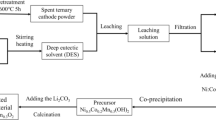
Abstract
As one of the main power sources, lithium-ion batteries (LIBs) will continue to be applied on a large scale in the future market. Commercialized LIBs cathode materials contain various valuable metal elements including Li, Co, and Ni. The development of green and efficient recycling technology is essential. Deep eutectic solvents (DESs) have recently emerged as a new class of green solvents that could potentially be applied for the LIBs recycling industry due to their capability to leach the active materials without the introduction of environmentally hazardous reagents as used in conventional hydrometallurgy process. Considering the rapid progress, a critical review focusing on the progress and fundamentals about the overall efficiency has practical significance for the further advancement of this field. Herein, we briefly introduce the overall process of using DESs for LIBs recycling and specifically focus on the efficiency of each step. Current fundamental understandings and new experimental approaches to enhance the efficiency of the leaching process, the extraction process, and reusability are summarized. We expect this review article to inspire scientists to use DESs as powerful tools for advancing the frontiers of LIBs recycling.


Copyright 2022, ACS Publications


Copyright 2006, ACS Publications. c Solubility of metal oxides in ptsa-ChCl type DES with different ratios. Reproduced with permission [93]. Copyright 2019, ACS Publications

Copyright 2019, Nature Publishing Group. d Possible delamination mechanism based on competitive inhibition of binding via hydrogen bonding. Reproduced with permission [95]. Copyright 2020, ChemSusChem

Copyright 2021, Green Chemistry. b The selection of DES with reductive property by calculating the atomic charges based on the Fukui function. Reproduced with permission [103]. Copyright 2020, Green Chemistry. c Evaluate the difficulty of capturing electrons based on IP calculation [104]. d The organic acid with a lower potential for its first oxidation was screened based on IP calculation. The DESs composed of ChCl as the HBA and various organic acid species as the HBD could be used to compare the leaching efficiency of NMC cathode material. Reproduced with permission [104]. Copyright 2021, Chemical Engineering Journal

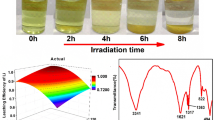
Copyright 2021, Journal of Kunming University of Science and Technology (Natural Science Edition). b The influence of the alcohol chain length for HBD on the viscosity. Reproduced with permission [119]. Copyright 2021, Elsevier. c The influence of the additional water content on the leaching process of Co and Li in ChCl-LAA DES. Reproduced with permission [104]. Copyright 2021, Chemical Engineering Journal. d GC–MS analysis of ChCl-EG DES heated at 180 °C for 24 h [121]. e Evolution of the XPS data for the metal oxide during the leaching process. The O1s spectra of the original electrode powder and the residual powder after leaching at 180 °C for 24 h show that the surface hydroxyl oxygen increases after leaching [121]. f UV–Vis spectrum shows the effect of N2 addition on the Co complexing ability [121]. g Comparison of the metal extraction yields of fresh and reused DESs. Reproduced with permission [121]. Copyright 2021, Chemical Engineering Journal

Copyright 2020, Green Chemistry. g–i shows the SEM images of the Ni-Co nuclei obtained on the GCE surface using electrodeposition by applying a constant potential of − 0.78 V νs. Ag QRE, during 300 s at different magnifications: g: 2500 × ; h: 10,000 × ; i: 50,000 × . Reproduced with permission [128]. Copyright 2020, Journal of Alloys and Compounds

Copyright 2022, Journal of Energy Chemistry. Comparison of the rate performances between b recycled and c commercial LNCO cathodes at different C rates. Reproduced with permission [50]. Copyright 2022, ChemSusChem
Similar content being viewed by others
References
Wang YQ et al (2021) Recent progress on the recycling technology of Li-ion batteries. J Energy Chem 55:391–419
Ghosh A (2020) Possibilities and challenges for the inclusion of the electric vehicle (EV) to reduce the carbon footprint in the transport sector: a review. Energies 13(10):2602
Kumar RR, Alok K (2020) Adoption of electric vehicle: a literature review and prospects for sustainability. J Clean Prod 253:119911
Santana IL et al (2017) Photocatalytic properties of Co3O4/LiCoO2 recycled from spent lithium-ion batteries using citric acid as leaching agent. Mater Chem Phys 190:38–44
Padwal C et al (2022) Deep eutectic solvents: green approach for cathode recycling of Li-ion batteries. Adv Energy Sustain Res 3(1):2100133
Zhao SQ et al (2021) Towards high-energy-density lithium-ion batteries: strategies for developing high-capacity lithium-rich cathode materials. Energy Storage Mat 34:716–734
Cheng J et al (2021) Recovery of cobalt from spent lithium-ion batteries as the dopant of TiO2 photocatalysts for boosting ciprofloxacin degradation. J Clean Prod 316:128279
Jyoti J, Singh BP, Tripathi SK (2021) Recent advancements in development of different cathode materials for rechargeable lithium ion batteries. J Energy Storage 43:103112
Liu J et al (2021) Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater Today 43:132–165
Wang W-Y, Yang H-C, Xu R-B (2020) High-performance recovery of cobalt and nickel from the cathode materials of NMC type li-ion battery by complexation-assisted solvent extraction. Minerals 10(8):662
Doose S et al (2021) Challenges in ecofriendly battery recycling and closed material cycles: a perspective on future lithium battery generations. Metals 11(2):291
Kim S et al (2021) A comprehensive review on the pretreatment process in lithium-ion battery recycling. J Clean Prod 294:126329
Sethurajan M, Gaydardzhiev S (2021) Bioprocessing of spent lithium ion batteries for critical metals recovery–a review. Resour Conserv Recycl 165:105225
Raj T et al (2022) Recycling of cathode material from spent lithium-ion batteries: challenges and future perspectives. J Hazard Mater 429:128312
Bai Y et al (2020) Energy and environmental aspects in recycling lithium-ion batteries: concept of battery identity global passport. Mater Today 41:304–315
Fan X et al (2021) Separation and recovery of valuable metals from spent lithium-ion batteries via concentrated sulfuric acid leaching and regeneration of LiNi1/3Co1/3Mn1/3O2. J Alloy Compd 863:158775
Fan X et al (2021) A green, efficient, closed-loop direct regeneration technology for reconstructing of the LiNi0.5Co0.2Mn0.3O2 cathode material from spent lithium-ion batteries. J Hazard Mater 410:124610
Yun L et al (2018) Metallurgical and mechanical methods for recycling of lithium-ion battery pack for electric vehicles. Resour Conserv Recycl 136:198–208
Verma A et al (2020) Separation of lithium and cobalt from LiCoO2: a unique critical metals recovery process utilizing oxalate chemistry. ACS Sustain Chem Eng 8(15):6100–6108
Sonoc A, Jeswiet J, Soo VK (2015) Opportunities to improve recycling of automotive lithium ion batteries. Procedia CIRP 29:752–757
Yao YL et al (2018) Hydrometallurgical processes for recycling spent lithium-ion batteries: a critical review. Acs Sustain Chem Eng 6(11):13611–13627
Li L et al (2013) Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J Power Sources 233:180–189
Nayaka GP et al (2016) Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag 51:234–238
Wang RH et al (2021) Synthesis of Li2FeP2O7 cathode material at different temperatures and its electrochemical performance for lithium ion batteries. Chem J Chin Universities-Chinese 42(4):1299–1306
Wang T et al (2020) Direct recycling of spent NCM cathodes through ionothermal lithiation. Adv Energy Mater 10(30):2001204
Ji Y, Jafvert CT, Zhao F (2021) Recovery of cathode materials from spent lithium-ion batteries using eutectic system of lithium compounds. Resour Conserv Recycl 170:105551
Baum ZJ et al (2022) Lithium-ion battery recycling─overview of techniques and trends. ACS Energy Lett 7(2):712–719
Choi J-W, Cho C-W, Yun Y-S (2022) Organic acid-based linear free energy relationship models for green leaching of strategic metals from spent lithium-ion batteries and improvement of leaching performance. J Hazard Mater 423:127214
Harper G et al (2019) Recycling lithium-ion batteries from electric vehicles. Nature 575(7781):75–86
Peeters N, Binnemans K, Riaño S (2020) Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem 22(13):4210–4221
Abbott AP et al (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun 1:70–71
Ge X et al (2017) Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: challenges, opportunities, and future vision. Journal of Materials Chemistry A 5(18):8209–8229
Abbott AP et al (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126(29):9142–9147
Tran MK et al (2019) Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat Energy 4(4):339–345
Hansen BB et al (2021) Deep eutectic solvents: a review of fundamentals and applications. Chem Rev 121(3):1232–1285
Wu J et al (2021) Deep eutectic solvents for boosting electrochemical energy storage and conversion: a review and perspective. Adv Func Mater 31(22):2011102
Zhang P et al (1998) Hydrometallurgical process for recovery of metal values from spent nickel-metal hydride secondary batteries. Hydrometallurgy 50(1):61–75
Lee CK, Rhee K-I (2003) Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 68(1–3):5–10
Joulié M, Laucournet R, Billy E (2014) Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J Power Sources 247:551–555
Thompson DL et al (2022) Separation of nickel from cobalt and manganese in lithium ion batteries using deep eutectic solvents. Green Chem. https://doi.org/10.1039/D2GC00606E
Lu B et al (2022) High-efficiency leaching of valuable metals from waste Li-ion batteries using deep eutectic solvents. Environ Res 212:113286
Li T et al (2022) Closed-loop cobalt recycling from spent lithium-ion batteries based on a deep eutectic solvent (DES) with easy solvent recovery. J Energy Chem. https://doi.org/10.1016/j.jechem
Yuan Z et al (2022) Status and advances of deep eutectic solvents for metal separation and recovery. Green Chem 24(5):1895–1929
Tome LI et al (2018) Deep eutectic solvents for the production and application of new materials. Appl Mater Today 10:30–50
Dwamena AK (2019) Recent advances in hydrophobic deep eutectic solvents for extraction. Separations 6(1):9
Inman G, Nlebedim IC, Prodius D (2022) Application of ionic liquids for the recycling and recovery of technologically critical and valuable metals. Energies 15(2):628
Fan M et al (2021) Progress in the sustainable recycling of spent lithium-ion batteries. SusMat 1(2):241–254
Du K et al (2022) Progresses in sustainable recycling technology of spent lithium-ion batteries. ENERGY ENVIRON MATER. https://doi.org/10.1002/eem2.12271
Neumann J et al (2022) Recycling of lithium‐ion batteries—current state of the art, circular economy, and next generation recycling. Adv Energy Mater 2102917
Morina R et al (2022) Cathode active material recycling from spent lithium batteries: a green (circular) approach based on deep eutectic solvents. Chemsuschem 15(2):e202102080
Chang X et al (2022) Selective extraction of transition metals from spent LiNixCoyMn1−x−yO2 cathode via regulation of coordination environment. Angew Chem Int Ed 61(24):e202202558
Abbott, A.P., et al. 2007 Eutectic‐based ionic liquids with metal‐containing anions and cations Chemistry–A European Journal 13(22):6495-6501
Yu D, Xue Z, Mu T (2021) Eutectics: formation, properties, and applications. Chem Soc Rev 50(15):8596–8638
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114(21):11060–11082
Chen Y, Mu T (2021) Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem Eng 2(2):174–186
Abbott AP, Capper G, Gray S (2006) Design of improved deep eutectic solvents using hole theory. ChemPhysChem 7(4):803–806
Abbott AP et al (2007) Eutectic-based ionic liquids with metal-containing anions and cations. Chemistry – A European Journal 13(22):6495–6501
Abbott AP (2005) Model for the conductivity of ionic liquids based on an infinite dilution of holes. ChemPhysChem 6(12):2502–2505
Hanada T, Goto M (2021) Synergistic deep eutectic solvents for lithium extraction. ACS Sustain Chem Eng 9(5):2152–2160
Zhang M et al (2022) Preparation strategy and stability of deep eutectic solvents: a case study based on choline chloride-carboxylic acid. J Clean Prod 345:131028
Huang F et al (2022) Ternary deep eutectic solvent (DES) with a regulated rate-determining step for efficient recycling of lithium cobalt oxide. ACS Omega 7(13):11452–11459
Chen Y et al (2021) Significant improvement in dissolving lithium-ion battery cathodes using novel deep eutectic solvents at low temperature. ACS Sustain Chem Eng 9(38):12940–12948
Cao J, Su E (2021) Hydrophobic deep eutectic solvents: the new generation of green solvents for diversified and colorful applications in green chemistry. J Clean Prod 314:127965
Milevskii N et al (2022) Separation of Li (I), Co (II), Ni (II), Mn (II), and Fe (III) from hydrochloric acid solution using a menthol-based hydrophobic deep eutectic solvent. Hydrometallurgy 207:105777
Amphlett J, Choi S (2021) The effect of increasing water content on transition metal speciation in deep eutectic solvents. J Mol Liq 332:115845
Popescu A-M, Constantin V (2014) Synthesis, characterization and thermophysical properties of three neoteric solvents-ionic liquids based on choline chloride. Chem Res Chin Univ 30(1):119–124
Jaumaux P et al (2020) Deep-eutectic-solvent-based self-healing polymer electrolyte for safe and long-life lithium-metal batteries. Angew Chem Int Ed 59(23):9134–9142
Angsantikul P et al (2021) Ionic liquids and deep eutectic solvents for enhanced delivery of antibodies in the gastrointestinal tract. Adv Func Mater 31(44):2002912
Pan Y et al (2022) Highly efficient absorption of HCl in deep eutectic solvents and their corresponding ethylene glycol blends. Chem Eng J 434:134707
Yang J et al (2021) Alkaline deep eutectic solvents as novel and effective pretreatment media for hemicellulose dissociation and enzymatic hydrolysis enhancement. Int J Biol Macromol 193:1610–1616
Dai YT et al (2015) Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem 187:14–19
Abbott AP, Capper G, Gray S (2006) Design of improved deep eutectic solvents using hole theory. Chemphyschem Eur J Chem Physics Physical Chem 7(4):803–806
Nkuku CA, LeSuer RJ (2007) Electrochemistry in deep eutectic solvents. J Phys Chem B 111(46):13271–13277
Gorke JT, Srienc F, Kazlauskas RJ (2008) Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem Commun 10:1235–1237
Morrison HG, Sun CC, Neervannan S (2009) Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int J Pharm 378(1–2):136–139
Gutiérrez Ma C, Rubio F, del Monte F (2010) Resorcinol-formaldehyde polycondensation in deep eutectic solvents for the preparation of carbons and carbon− carbon nanotube composite. Chem Mater 22(9):2711–2719
Zhao H, Baker GA, Holmes S (2011) Protease activation in glycerol-based deep eutectic solvents. J Mol Catal B Enzym 72(3–4):163–167
Abbott AP et al (2012) The electrodeposition of silver composites using deep eutectic solvents. Phys Chem Chem Phys 14(7):2443–2449
Oliveira FS et al (2013) Deep eutectic solvents as extraction media for azeotropic mixtures. Green Chem 15(5):1326–1330
Zhou E, Liu H (2014) A novel deep eutectic solvents synthesized by solid organic compounds and its application on dissolution for cellulose. Asian J Chem 26(12):3626–3630
Duan L et al (2016) Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain ChemEng 4(4):2405–2411
Kumar AK, Parikh BS, Cotta MA (2018) Application of natural deep eutectic solvents in biomass pretreatment, enzymatic saccharification and cellulosic ethanol production. Mater Today: Proc 5(11):23057–23063
Emami S, Shayanfar A (2020) Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm Dev Technol 25(7):779–796
Kalhor P, Ghandi K (2021) Deep eutectic solvents as catalysts for upgrading biomass. Catalysts 11(2):178
Zhang Q et al (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41(21):7108–7146
Choi YH et al (2011) Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol 156(4):1701–1705
Imperato G et al (2005) Low-melting sugar-urea-salt mixtures as solvents for Diels-Alder reactions. Chem Commun (Camb) 9:1170–1172
Germani R et al (2017) Novel low viscous, green and amphiphilic N -oxides/phenylacetic acid based deep eutectic solvents. J Mol Liq 240:233–239
Goeltz JC, Matsushima LN (2017) Metal-free redox active deep eutectic solvents. Chem Commun 53(72):9983–9985
Richter J, Ruck M (2020) Synthesis and dissolution of metal oxides in ionic liquids and deep eutectic solvents. Molecules 25(1):78
Abbott AP et al (2006) Solubility of metal oxides in deep eutectic solvents based on choline chloride. J Chem Eng Data 51(4):1280–1282
Abbasi NM, Farooq MQ, Anderson JL (2021) Investigating the variation in solvation interactions of choline chloride-based deep eutectic solvents formed using different hydrogen bond donors. ACS Sustain Chem Eng 9(35):11970–11980
Rodriguez N, Machiels L, Binnemans K (2019) p-Toluenesulfonic acid-based deep-eutectic solvents for solubilizing metal oxides. ACS Sustainab Chem Eng 7(4):3940–3948
Laso J et al (2015) Selective recovery of zinc over iron from spent pickling wastes by different membrane-based solvent extraction process configurations. Ind Eng Chem Res 54(12):3218–3224
Bai Y et al (2020) Sustainable direct recycling of lithium-ion batteries via solvent recovery of electrode materials. Chemsuschem 13(21):5664–5670
Wang M et al (2019) A low-toxicity and high-efficiency deep eutectic solvent for the separation of aluminum foil and cathode materials from spent lithium-ion batteries. J Hazard Mater 380:120846
Hsieh YT et al (2014) Speciation of cobalt-chloride-based ionic liquids and electrodeposition of Co wires. Electrochim Acta 117:217–223
Fu YP et al (2019) Effective leaching and extraction of valuable metals from electrode material of spent lithium-ion batteries using mixed organic acids leachant. J Ind Eng Chem 79:154–162
Roldán-Ruiz MJ et al (2020) Highly efficient p-Toluenesulfonic acid-based deep-eutectic solvents for cathode recycling of Li-ion batteries. ACS Sustain Chem Eng 8(14):5437–5445
Fu Y et al (2020) Microwave reduction enhanced leaching of valuable metals from spent lithium-ion batteries. J Alloy Compd 832:154920
Xu ZW et al (2021) Use of Microwave-assisted deep eutectic solvents to recycle lithium manganese oxide from Li-ion batteries. Jom 73(7):2104–2110
Chen LL et al (2021) Engineering a tandem leaching system for the highly selective recycling of valuable metals from spent Li-ion batteries. Green Chem 23(5):2177–2184
Wang S et al (2020) A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chem 22(14):4473–4482
Hua Y, et al. 2021 Ionization potential-based design of deep eutectic solvent for recycling of spent lithium ion batteries. Cheml Eng J 133200
Elgrishi N et al (2017) A practical beginner’s guide to cyclic voltammetry. J Chem Educ 95(2):197–206
Carriedo GA (1988) The use of cyclic voltammetry in the study of the chemistry of metal-carbonyls: an introductory experiment. J Chem Educ 65(11):1020
Smith JR, Campbell SA, Walsh FC (1995) Cyclic voltammetry at metal electrodes. Transac IMF 73(2):72–78
Zhang XX et al (2018) Innovative application of acid leaching to regenerate Li(Ni1/3Co1/3Mn1/3)O-2 cathodes from spent lithium-ion batteries. Acs Sustain Chem Eng 6(5):5959–5968
Yao L et al (2016) Recycling and synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries using D. L-malic acid Rsc Advances 6(22):17947–17954
Hannan MA et al (2017) Review of energy storage systems for electric vehicle applications: issues and challenges. Renew Sustain Energy Rev 69:771–789
Li Y, Song J, Yang J (2014) A review on structure model and energy system design of lithium-ion battery in renewable energy vehicle. Renew Sustain Energy Rev 37:627–633
Wang S et al (2020) Reduction-ammoniacal leaching to recycle lithium, cobalt, and nickel from spent lithium-ion batteries with a hydrothermal method: effect of reductants and ammonium salts. Waste Manag 102:122–130
Or T et al (2020) Recycling of mixed cathode lithium-ion batteries for electric vehicles: current status and future outlook. Carbon Energy 2(1):6–43
Tang S, Zhang M, Guo M (2022) A novel deep-eutectic solvent with strong coordination ability and low viscosity for efficient extraction of valuable metals from spent lithium-ion batteries. ACS Sustai Chem Eng 10(2):975–985
Tang J et al (2018) Anodic dissolution of copper in choline chloride-urea deep eutectic solvent. J Electrochem Soc 165(9):E406–E411
Doche M-L et al (2017) An ultrasonic-assisted process for copper recovery in a des solvent: leaching and re-deposition. Chem Eng Process 121:90–96
Zhu X-L et al (2019) Selective recovery of zinc from zinc oxide dust using choline chloride based deep eutectic solvents. Trans Nonferrous Met Soc Chin 29(10):2222–2228
Hammond OS et al (2017) Resilience of malic acid natural deep eutectic solvent nanostructure to solidification and hydration. J Phys Chem B 121(31):7473–7483
Cotroneo-Figueroa VP et al (2022) Hydrogen bond donor and alcohol chain length effect on the physicochemical properties of choline chloride based deep eutectic solvents mixed with alcohols. J Mol Liq 345:116986
Zhang XJ, et al.2016Physicochemical properties of ChCl-EG deep eutectic solvent Journal of Kunming University of Science and Technology ( Natural Science Edition),. 41(02): p. 8-14
Schiavi PG et al (2021) Selective recovery of cobalt from mixed lithium ion battery wastes using deep eutectic solvent. Chem Eng J 417:129249
Vicente JDS et al (2021) On the dissolution of metals in ionic liquids 1. iron cobalt, nickel, copper, and zinc. Sustainable Chemistry 2(1):63–73
Eaton DR, O’Reilly A (1987) Oxidation of cobalt (II) amine complexes to mononuclear cobalt (III) complexes by dioxygen. Inorg Chem 26(25):4185–4188
Pateli IM et al (2020) The effect of pH and hydrogen bond donor on the dissolution of metal oxides in deep eutectic solvents. Green Chem 22(16):5476–5486
Söldner A, Zach J, König B (2019) Deep eutectic solvents as extraction media for metal salts and oxides exemplarily shown for phosphates from incinerated sewage sludge ash. Green Chem 21(2):321–328
Gao Z et al (2017) Ultrathin Mg-Al layered double hydroxide prepared by ionothermal synthesis in a deep eutectic solvent for highly effective boron removal. Chem Eng J 319:108–118
Pegoretti VCB et al (2017) Thermal synthesis, characterization and electrochemical study of high-temperature (HT) LiCoO2 obtained from Co(OH)(2) recycled of spent lithium ion batteries. Mater Res Bull 86:5–9
Landa-Castro M et al (2020) Ni–Co alloy electrodeposition from the cathode powder of Ni-MH spent batteries leached with a deep eutectic solvent (reline). J Alloy Compd 830:154650
Chen W et al (2021) Tailoring hydrophobic deep eutectic solvent for selective lithium recovery from the mother liquor of Li2CO3. Chem Eng J 420:127648
Acknowledgements
This work was supported by the Natural Science Foundation of Guizhou Science and Technology Department (QKHJC-ZK[2021]-YB257) and the Talent program of Guizhou University (702759203301).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Li, S., Li, T. et al. Deep Eutectic Solvents (DESs) for Green Recycling of Wasted Lithium-Ion Batteries (LIBs): Progress on Pushing the Overall Efficiency. Mining, Metallurgy & Exploration 39, 2149–2165 (2022). https://doi.org/10.1007/s42461-022-00660-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-022-00660-7