Abstract
Purpose
Nano-sized biopolymer-based strategies such as spatio-temporal co-delivery of bioactives could be employed to overcome low bioavailability of antimalarial drugs delivered by conventional dosage forms in malaria combo-therapy. This work reports on the development of core–shell nanocarrier using a biocomposite containing Prosopis africana gum (PG) and microcrystalline cellulose (MCC) for spatio-temporal delivery of artemether and lumefantrine (AL) to enhance oral malaria treatment.
Methods
The biocomposite was prepared by pH-dependent temperature-controlled coacervation and characterized by differential scanning calorimetry, thermogravimetric measurements, and time-domain nuclear magnetic resonance relaxometry. Nanocarrier containing AL was produced using the combination of high-shear homogenization and nanoprecipitation techniques, characterized regarding physicochemical performance, in-vitro dissolution and stability. In-vivo studies were performed in mice infected with Plasmodium berghei parasite.
Results
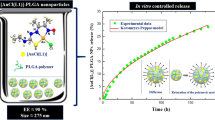
Biocomposite containing 1:1 ratio of PG:MCC gave the best physicochemical properties. The nanoparticles were spherical with a smooth surface, gave encapsulation efficiency of 81.23 ± 1.54% and 57.15 ± 0.86% for artemether and lumefantrine, respectively, and exhibited an average size of 208.29 ± 0.94 nm, a low polydispersity index value (0.141 ± 0.02) indicating a narrow size distribution and a positive charge of 34.51 ± 1.05 mV. The nanoparticles provided a quick release for artemether and a slow release for lumefantrine within 8 h, indicating the suitability for spatio-temporal drug delivery in malaria combo-therapy. Optimized AL-loaded nanoformulation was stable, histopathologically safe on major organs implicated in malaria and exhibited greater antimalarial activity than marketed AL.
Conclusion
These results postulate the developed core–shell nanocarrier as versatile drug co-delivery nanoplatform and potential alternative approach to improve the pharmacodynamics of AL combo-therapies in malaria treatment.












Similar content being viewed by others
References
Uduma EO, Ming H. Bioavailability and pharmacokinetics of dihydroartemisinin (DHA) and its analogs—mechanistic studies on its ADME. Curr Pharmacol Reports. 2018; 33–44.
Wangdi KL, Furuya-Kanamori J, Clark JJ, Barendregt ML, Gatton C, Banwell GC, Kelly SAR, Doi ACA. Comparative effectiveness of malaria prevention measures: a systematic review and network meta-analysis. Parasites & Vectors. 2018;11:1–13.
World Health Organization. World Malaria Report. Geneva, Switzerland: World Health Organization; 2018.
Nnamani PO, Ugwu AA, Ibezim EC, Kenechukwu FC, Akpa PA, Ogbonna JDN, Obitte NC, Odo A, Windbergs M, Lehr CM, Attama AA. Sustained-release liquisolid compact tablets containing artemether-lumefantrine as alternate-day regimen for malaria treatment to improve patient compliance. Int J Nanomed. 2016;11:6365–78.
Pelfrene E, Pinheiro M-H, Cavaleri M. Artemisinin-based combination therapy in the treatment of uncomplicated malaria: review of recent regulatory experience at the European Medicines Agency. Int Health. 2015;7:239–46.
Amin CN, Fabre H, Blanchin M. Determination of artemether and lumefantrine in anti-malarial fixed–dose combination tablets by microemulsion electrokinetic chromatography with short-end injection procedure. Malaria J. 2013;12:202.
Novatis Pharma Ag. Coartem (artemether/lumefantrine) tablet description. Available at https://www.rxlist.com/caortem-drug.htm. Accessed on 29 Sept 2018.
Parashar D, Aditya NP, Murthy RSR. Development of artemether and lumefantrine co-loaded nanostructured lipid carriers: physicochemical characterization and in vivo antimalarial activity. Drug Deliv. 2014;23:1–7.
Aditya NP, Patankar S, Madhusudhan B, Murthy RS, Souto EB. Artemether loaded lipid nanoparticles produced by modified thin-film hydration: pharmacokinetics, toxicological and in vivo anti-malarial activity. Eur J Pharm Sci. 2010;40:448–55.
Duchêne D, Gref R. Small is beautiful: surprising nanoparticles. Int J Pharm. 2016;502:219–31.
Umeyor CE, Kenechukwu FC, Uronnachi EM, Chime SA, Reginald-Opara J, Attama AA. Recent advances in particulate anti-malarial drug delivery systems: a review. Int J Drug Deliv. 2013;5:1–14.
Zhang L, Wang S, Zhang M, Sun J. Nanocarriers for oral drug delivery. J Drug Target. 2013;21:515–27.
Chaires-Martínez L, Salazar-Montoya JA, Ramos-Ramírez EG. Physicochemical and functional characterization of the galactomannan obtained from mesquite seeds (Prosopis pallida). Eur Food Res Technol. 2008;227:1669–76.
Builders PF, Ibekwe N, Okpako LC, Attama AA, Kunle OO. Preparation and characterization of mucinated cellulose microparticles for therapeutic and drug delivery purposes. Eur J Pharm Biopharm. 2009;72:34–41.
Builders PF, Agbo MB, Tiwalade A, Okpako LC, Attama AA. Novel multifunctional pharmaceutical excipients derived from microcrystalline cellulose–starch microparticulate composites prepared by compatibilized reactive polymer blending. Int J Pharm. 2010;388:159–67.
Nnamani PO, Hansen S, Windbergs M, Lehr C-M. Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application. Int J Pharm. 2014;477:208–17.
Adikwu MU, Yoshikawa Y, Takada K. Bioadhesive delivery of metformin using prosopis gum with antidiabetic potential. Biol Pharm Bull. 2003;26:662–6.
Nnamani PO, Kenechukwu FC, Okoye O, Akpa PA. Sustained-release mebendazole microcapsules prepared with Prosopis africana peel powder (PAPP) hydrogel. Ind J Novel Drug Deliv. 2017;9:167–84.
Nadaf S, Nnamani PO, Jadhav N. Evaluation of Prospis africana seed gum as an extended release polymer for tablet formulation. AAPS Pharm Sci Technol. 2015;16:716–28.
Ummartyotin S, Pechyen C. Microcrystalline-cellulose and polypropylene based composite: a simple, selective and effective material for microwavable packaging. Carbohydr Polym. 2016;142:133–40.
Nasution H, Veronicha Y, Irmadani S, Sitompul S. Preparation and characterization of cellulose microcrystalline (MCC) from fiber of empty fruit bunch palm oil, 1st Annual Applied Science and Engineering Conference. IOP Conference Series: Materials Science and Engineering, 2017;180:1–8. 012007. https://doi.org/10.1088/1757-899X/180/1/012007.
Kenechukwu FC, Dias ML, Ricci-Júnior E. Biodegradable nanoparticles from prosopisylated cellulose as a platform for enhanced oral bioavailability of poorly water-soluble drugs. Carbohydr Polym. 2020;256:1–13. https://doi.org/10.1016/j.carbpol.2020.117492.
Xu S, Yi S, He J, Wang H, Fang Y, Wang Q. Preparation and properties of a novel microcrystalline cellulose-filled composites based on polyamide 6/high-density polyethylene. Materials. 2017;10:1–12.
Dias ML, Dip RMM, Souza DHS, Nascimento JP, Santos AP, Furtado CA. Electrospun nanofibers of poly(lactic acid)/ graphene nanocomposites. J Nanosci Nanotechnol. 2017;17:2531–40.
Gao Y, Zhang R, Lv W, Liu Q, Wang X, Sun P, Winter HH, Xue G. Critical effect of segmental dynamics in polybutadiene/clay nanocomposites characterized by solid state 1H NMR spectroscopy. J Phys Chem C. 2014;118:5606–14.
Neto RPC, Da Rocha R, Elton J, Tavares MIB. Proton NMR relaxometry as probe of gelatinization, plasticization and montmorillonite-loading effects on starch-based materials. Carbohydr Polym. 2018;182:123–31.
Cheung TTP, Gerstein BC. 1H nuclear magnetic resonance studies of domain structures in polymers. J Appl Physics. 1981;52:5517–28.
Kenechukwu FC, Attama AA, Ibezim EC, Nnamani PO, Umeyor CE, Uronnachi EM, Gugu TH, Momoh MA, Ofokansi KC, Akpa PA. Surface-modified mucoadhesive microgels as a controlled release system for miconazole nitrate to improve localized treatment of vulvovaginal candidiasis. Eur J Pharm Sci. 2018;111:358–75. https://doi.org/10.1016/j.ejps.2017.10.002.
Lopes M, Shrestha N, Correia A, Shahbazi MA, Sarmento B, Hirvonen J, Veiga F, Seica R, Ribeiro A, Santos HA. Dual chitosan/albumin-coated alginate/ dextran sulfate nanoparticles for enhanced oral delivery of insulin. J Control Rel. 2016;23:29–41. https://doi.org/10.1016/j.jconrel.2016.04.012.
Patel K, Sarma vvp. Design and evaluation of lumefantrine-oleic acid self nano emulsifying ionic complex for enhanced dissolution. Daru 2013;21-27 https://doi.org/10:1186/2008-2231-21-7
Agubata CO, Nzekwe IT, Attama AA, Mueller-Goymann CC, Onunkwo GC. Formulation, characterization and anti-malarial activity of homolipid-based artemether microparticles. Int J Pharm. 2015;478:202–22.
Kamal AH, EL-Malla SF, Hammad SF. A review on UV spectrophotometric methods for simultaneous multicomponent analysis. Eur J Pharm Med Res. 2016;2:348–360.
Afosah D. Design of simple UV spectrophotometric and HPLC methods for the assay of artemether and lumefantrine in fixed-dose combination tablets. MSc Thesis, Kwame Nkrumah University of Science and Technology, Kumasi. (2010) Available from: https://dspace.knust.edu.gh:8080/jspui/bitstream/123456789/239/1/DANIEL%20AFOSAH%20%28THESIS%29.pdf.http://hdl.handle.net/123456789/356 [last Accessed 8 Aug 2019].
Kuideep V, Neelam B, Sheetal M, Jain D. Formulation and evaluation of tablets containing artemether microspheres and lumefantrine. J Drug Deliv Therap. 2017;7:4–7.
Prasanna R, Saravanan D, Padmavathy J. Method development and validation for the determination of lumefantrine in sold dosage form by RP-HPLC. Int J Pharm Res Dev. 2010;8:84–90.
Christian J, Shah P, Patel M, Patel K, Gandhi T. Optimizing derivatization conditions using an experimental design and simultaneous estimation of artemether and lumefantrine by ratio first order derivative spectrophotometric method. J Taibah Univ Sci. 2017;11:729–40.
Kalyankar TM, Attar MS, Deosarkar SD. Development of UV-visible spectrophotometric method for simultaneous estimation of artemether and lumefantrine. Anal Chem. 2013;12:83–8.
Beckett AH, Stenlake JB. Practical pharmaceutical chemistry. Part II, 4th. ed., London; Bloomsbury Publishing 2001.
Attama AA, Ayogu IJ, Kenechukwu FC, Ogbonna JDN, Okore VC. A new lipid-based drug delivery system (LBDDS) for oral delivery of tioconazole. Int J Drug Deliv. 2011;3:743–53.
Coartem (artemether/lumefantrine). https://www.medilexicon.com/drugs/coartem.php (Assessed Aug 2019).
Onwurah NE. Introductory biochemistry for life sciences. 1st ed. Enugu, Nigeria: Immaculate Pub. Ltd.; 2001. p. 21–49.
Agbo CP, Umeyor CE, Kenechukwu FC, Ogbonna JDN, Chime SA, Charles L, Agubata CO, Ofokansi KC, Attama AA. Formulation design, in vitro characterizations and anti-malarial investigations of artemether and lumefantrine-entrapped solid lipid microparticles. Drug Dev Ind Pharm. 2016;42:1708–21. https://doi.org/10.3109/03639045.2016.1171331.
Vendruscolo CW, Ferrero C, Pineda EAG, Silveira JLM, Freitas RA, Jiménez-Castellanos EAG, Bresolin TMB. Physicochemical and mechanical characterization of galactomannan from Mimosa scabrella: effect of drying method. Carbohydr Polym. 2009;76:86–93.
Mendes JF, Paschoalin RT, Carmona VB, Neto ARS, Marques ACP, Marconcini JM, Mattoso LHC, Medeiros ES, Oliveira JE. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr Polym. 2016;137:452–8.
Siddiqui MZ, Prasad N, Tewari JC. Physicochemical properties and toxicity test of Prosopis juliflora (Sw.) DC. and Balanites aegyptiaca Del gum exudates from Rajasthan. Ind J Exp Biol. 2017;55:782–788.
Kong L, You L, Feng T, Yin X, Liu T, Dong L. Physicochemical characterization of the polysaccharide from Bletilla striata: effect of drying method. Carbohydr Polym. 2015;125:1–8.
Builders PF, Anwunobi PA, Mbah CC, Adikwu MU. New direct compression excipient from tigernut starch: physicochemical and functional properties. AAPS Pharm Sci Tech. 2013;14:818–27.
Attama AA, Akpa PA. Determination of the amorphicity and glass transition temperatures (Tg) of some natural polysaccharides. J Drug Deliv Sci Technol. 2008;18:219–20.
Thakur VK, Thakur MK, Gupta RK. Rapid synthesis of graft copolymers from natural cellulose fibers. Carbohydr Polym. 2013;98:820–8.
Momoh MA, Kenechukwu FC, Ofokansi KC, Attama AA, Díaz DD. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr Polym. 2020;229:1–10. https://doi.org/10.1016/j.carbpol.2019.115506.
Attama AA, Kenechukwu FC, Onuigbo EB, Nnamani PO, Obitte NC, Finke JH, Pretor S, Muller-Goymann CC. Solid lipid nanoparticles encapsulating a fluorescent marker (coumarin 6) and anti-malarials – artemether and lumefantrine: evaluation of cellular uptake and anti-malarial activity. Eur J Nanomed. 2016;8:129–138
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51.
Yahaya ZS, Ofokansi KC, Allagh ST, Bhatia PG. Preparation and characterization of artemether inclusion complexes with hydroxypropyl-β-cyclodextrin. Trop J Pharm Res. 2017;16:2359–64.
Trasi NS, Bhujbal SV, Zemlyanov DY, Zhou QT, Taylor LS. Physical stability and release properties of lumefantrine amorphous solid dispersion granules prepared by a simple solvent evaporation approach. Int J Pharm X 2020;2:1–13.
Fule RA, Meer TS, Sav AR, Amin PD. Dissolution rate enhancement and physicochemical characterization of artemether and lumefantrine solid dispersions. Int J Drug Deliv. 2012;4:95–106.
Saini S, Singh G, Kalara N, Singh C, Virmani GT. Development of nanostructured liquid crystalline formulation of antimalarial agents: artemether and lumefantrine. World J Pharm Sci. 2015;4:1811–28.
Awofisayo SO, Arhewoh M, Okhamafe AO. In vitro interaction studies between artemether – lumefantrine and lamivudine/metronidazole. Int J Curr Res Rev. 2018;10:25–30.
Bravo Gonza´lez RC, Huwyler J, Boess F, Walter I, Bittner B. In vitro investigation on the impact of the surface-active excipients - cremophor EL, Tween 80 and Solutol HS 15 on the metabolism of midazolam. Biopharm Drug Disp. 2004;25:37–49.
Christiansen A, Backensfeld T, Denner K, Weitschies W. Effects of non-ionic surfactants on cytochrome P450-mediated metabolism in vitro. Eur J Pharm Biopharm. 2011;78:166–72.
Deepa P, Aditya NP, Murthy RRS. Development of artemether and lumefantrine co-loaded nanostructured lipid carriers: physicochemical characterization and in vivo antimalarial activity. Drug Deliv. 2016;23:123–9. https://doi.org/10.3109/10717544.2014.905883.
Priyanka P, Narsee M. Artemether-lumefantrine nanostructured lipid carriers for oral malaria therapy: Enhanced efficacy at reduced dose and dosing frequency. Int J Pharm. 2015;511:473–87. https://doi.org/10.1016/j.ijpharm.2016.07.021.
Priyanka P, Shital S, Sulabha P, Aditua P, Shobhona P, Vandana P. Nanostructured lipid carriers for artemether-lumefantrine combination for intravenous therapy of cerebral malaria. Int J Pharm. 2016;513:504–17.
Yufan M, Tingli L, Wen Z, Ying W, Ting C, Oibing M, Tao C. Enhanced antimalarial activity by a novel artemether-lumefantrine lipid emulsion for parenteral administration. Antimicrob Agents Chemother. 2014;58:5658–5665. https://doi.org/10.1128/AAC.01428-13
Shende P, Desai P, Gaud R, Dhumatkar R. Engineering of microcomplex of artemether and lumefantrine for effective drug treatment in malaria. Artif Cells Nanomed Biotechnol. 2017;45:1597–604. https://doi.org/10.1080/21691401.2016.1267012.
Lefèvre G, Thomsen MS. Clinical pharmacokinetics of artemether and lumefantrine (Riamet®). Clin Drug Investig. 1999;18:467–80.
Wahajuddin R, Raju KSR, Singh SP, Taneja I. Investigation of the functional role of P-glycoprotein in limiting the oral bioavailability of lumefantrine. Antimicrob Agents Chemother. 2014;58:489–94.
Mountfield RJ, Senepin S, Schleimer M, Walter I, Bittner B. Potential inhibitory effects of formulation ingredients on intestinal cytochrome P450. Int J Pharm. 2000;211:89–92.
Coon JS, Knudson W, Clodfelter Lu KB, Weinstein RS. Solutol HS 15, nontoxic polyoxyethylene esters of 12-hydroxystearic acid, reverses multidrug resistance. Cancer Res. 1991;51:897–902.
Kumaratilake LM, Robinson BS, Ferrante A, Poulos A. Antimalarial properties of n-3 and n-6 polyunsaturated fatty acids; in vitro effects on Plasmodium falciparum and in vivo effects on P. berghei. J Clin Investig. 1999;89:961–967.
Krugliak M, Deharo E, Shalmiev G, Sauvain M, Moretti C, Ginsburg H. Antimalarial effects of C18 fatty acids on Plasmodium falciparum in culture and on Plasmodium vinckei petteri and Plasmodium yoelii nigeriensis in vivo. Experimen Parasitol. 1995;81:97–105.
Ogbonna JDN, Kenechukwu FC, Nwobi CS, Onochie SC, Attama AA. Formulation, in vitro and in vivo evaluation of halofantrine-loaded solid lipid microparticles. Pharm Dev Technol. 2015;20:941–8.
Ogbonna JDN, Nzekwe IT, Kenechukwu FC, Nwobi CS, Amah JI, Attama AA. Development and evaluation of chloroquine phosphate microparticles using solid lipid as a delivery carrier. J Drug Discov Dev Deliv. 2015;2:1011.
Acknowledgements
Dr. Franklin Chimaobi Kenechukwu greatly appreciates the mentorship of Prof. Dr. Marcos Lopes Dias and his research team for providing a good working condition for this research. The various contributions of the laboratory staff of Instituto de Macromoléculas Professora Eloisa Mano (IMA) and Nanomedicines Unit, Facultade de Pharmacia, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro-RJ, Brazil, are highly acknowledged.
Funding
The present work has been done with the financial support from the Brazilian National Council for Scientific and Technological Development (CNPq) — Brazil and The World Academy of Sciences (TWAS) — for the advancement of science in developing countries (Italy) (Grant FR number: 3240299288). This research work received support from the Tertiary Education Trust Fund (TETFund) (Grant No. TETFUND/DR&D/CE/NRF/2019/STI/46/) by Government of Nigeria.
Author information
Authors and Affiliations
Contributions
Franklin Chimaobi Kenechukwu: conceptualization, methodology, validation, resources, formal analysis, investigation, funding acquisition, writing — original and draft as well as revision. Marcos Lopes Dias: conceptualization, methodology, validation, resources, funding acquisition, supervision, project administration, writing — review and editing. Roberto Pinto Cucinelli Neto: conceptualization, methodology, validation, resources, formal analysis, investigation, writing. Eduardo Ricci-Júnior: conceptualization, methodology, validation, resources, supervision, writing – review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kenechukwu, F.C., Dias, M.L., Neto, R.P.C. et al. Compatibilized Biopolymer-based Core–shell Nanoparticles: A New Frontier in Malaria Combo-therapy. J Pharm Innov 18, 594–620 (2023). https://doi.org/10.1007/s12247-022-09664-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09664-8