Abstract
Purpose
This study aims to determine the effect of ultrasonic treatment and additive on the morphology, physicochemical properties, and dissolution of ibuprofen crystals.
Methods
Ultrasound-assisted anti-solvent crystallization was used to form ibuprofen crystals. The produced crystals were tested for various physicochemical properties.
Results
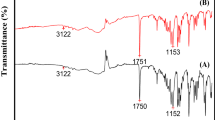
The morphology of the ibuprofen crystals was significantly altered in terms of size and shape after the anti-solvent crystallization process and ultrasonic treatment. The melting range of ibuprofen remained unaltered when measured via differential scanning calorimetry. The powder X-ray diffractometry analysis confirmed the presence of ibuprofen in a crystalline state. The anti-solvent addition at various times following ultrasonic treatment had no significant effect on drug dissolution. However, the polyethylene glycol (PEG) 400 and polysorbate 80 additives increased drug dissolution. The addition of 20% w/w PEG or polysorbate 80 resulted in higher drug dissolution than 10% w/w PEG or polysorbate 80.
Conclusion
The results demonstrate that the ultrasound-assisted crystallization of the anti-solvent with an appropriate duration affected the physical and chemical properties of treated ibuprofen crystals, making them more soluble, particularly when polysorbate 80 was used.







Similar content being viewed by others
References
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60. https://doi.org/10.1016/S0939-6411(00)00076-X.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. https://doi.org/10.1023/A:1016212804288.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. Int Sch Res Notices. 2012. https://doi.org/10.5402/2012/195727.
Javadzadeh Y, Hamedeyazdan S, Asnaashari S. Recrystallization of drugs: significance on pharmaceutical processing. Recrystallization. London: InTech. 2012:425–46. https://doi.org/10.5402/2012/195727
Lee J, Ashokkumar M, Kentish SE. Influence of mixing and ultrasound frequency on antisolvent crystallisation of sodium chloride. Ultrason Sonochem. 2014;21:60–8. https://doi.org/10.1016/j.ultsonch.2013.07.005.
De Castro ML, Priego-Capote F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason Sonochem. 2007;14:717–24. https://doi.org/10.1016/j.ultsonch.2006.12.004.
Todres ZV. Organic mechanochemistry and its practical applications. Florida: CRC Press; 2006.
USP Convention. US Pharmacopeia National Formulary 2017: USP 40 NF 35. United States Pharmacopeial USA. 2017.
Shah VP. The role of dissolution testing in the regulation of pharmaceuticals: the FDA perspective. Pharm Dissolution Test. Florida: CRC Press. 2005:99–114.
Nayhouse M, Tran A, Kwon JSI, Crose M, Orkoulas G, Christofides PD. Modeling and control of ibuprofen crystal growth and size distribution. Chem Eng Sci. 2015;134:414–22. https://doi.org/10.1016/j.ces.2015.05.033.
Li H, Wang J, Bao Y, Guo Z, Zhang M. Rapid sonocrystallization in the salting-out process. J Cryst Growth. 2003;247:192–8. https://doi.org/10.1016/S0022-0248(02)01941-3.
Kaialy W, Larhrib H, Chikwanha B, Shojaee S, Nokhodchi A. An approach to engineer paracetamol crystals by antisolvent crystallization technique in presence of various additives for direct compression. Int J Pharm. 2014;464:53–64. https://doi.org/10.1016/j.ijpharm.2014.01.026.
Zhu Q, Harris MT, Taylor LS. Modification of crystallization behavior in drug/polyethylene glycol solid dispersions. Mol Pharm. 2012;9:546–53. https://doi.org/10.1021/mp200546p.
Chen J, Ormes JD, Higgins JD, Taylor LS. Impact of surfactants on the crystallization of aqueous suspensions of celecoxib amorphous solid dispersion spray dried particles. Mol Pharm. 2015;12:533–41. https://doi.org/10.1021/mp5006245.
Rasenack N, Müller BW. Ibuprofen crystals with optimized properties. Int J Pharm. 2002;245:9–24. https://doi.org/10.1016/S0378-5173(02)00294-6.
Huanbutta K, Terada K, Sriamornsak P, Nunthanid J. Simultaneous X-ray diffraction-differential scanning calorimetry and physicochemical characterizations of spray dried drugs and chitosan microspheres. Walailak J Sci Technol. 2016;13:849–61.
Pohar A, Likozar B. Dissolution, nucleation, crystal growth, crystal aggregation, and particle breakage of amlodipine salts: modeling crystallization kinetics and thermodynamic equilibrium, scale-up, and optimization. Ind Eng Chem Res. 2014;53:10762–74. https://doi.org/10.1021/ie501572h.
Echigo T, Aruguete DM, Murayama M, Hochella MF Jr. Influence of size, morphology, surface structure, and aggregation state on reductive dissolution of hematite nanoparticles with ascorbic acid. Geochim Cosmochim Acta. 2012;90:149–62. https://doi.org/10.1016/j.gca.2012.05.008.
Savaroglu G, Caglar M, Ildaser AC, Hür E, Ilican S. The effect of sonication time on the surface morphology and dissolubility of naproxen sodium powders. Appl Surf Sci. 2019;492:66–72. https://doi.org/10.1016/j.apsusc.2019.06.165.
Hattori Y, Haruna Y, Otsuka M. Dissolution process analysis using model-free Noyes-Whitney integral equation. Colloids Surf B: Biointerfaces. 2013;102:227–31. https://doi.org/10.1016/j.colsurfb.2012.08.017.
Acknowledgements
We gratefully acknowledge financial support from the Royal Golden Jubilee Ph.D. Program, Thailand. Thanks to Waraporn Kuyukham, Karakadaporn Pimnoo, and Pannin Lapkern for performing preliminary experiments.
Funding
The study received financial support from the Royal Golden Jubilee PhD program, Thailand.
Author information
Authors and Affiliations
Contributions
The study conception and design were performed by Pornsak Sriamornsak and Rattanawich Minphimai. Material preparation, data collection, and analysis were performed by Kampanart Huanbutta, Rattanawich Minphimai, Suchada Piriyaprasarth, Sontaya Limmatvapirat, and Pornsak Sriamornsak. The first draft of the manuscript was written by Kampanart Huanbutta, Tanikan Sangnim, and Pornsak Sriamornsak, and all the authors commented on the previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huanbutta, K., Sangnim, T., Minphimai, R. et al. Ultrasound-Assisted Anti-Solvent Crystallization of Ibuprofen: Effect of Ultrasonic Treatment and Additive. J Pharm Innov 18, 575–584 (2023). https://doi.org/10.1007/s12247-022-09674-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09674-6