Abstract
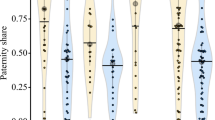
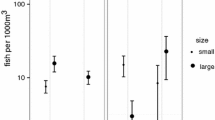
Size-assortative pairing is common across a wide range of taxa. In many cases, both sexes would benefit from pairing with a mate larger than themselves. As males and females cannot simultaneously be larger than their pair mate, size differences within pairs reflect which sex is able to obtain this benefit. Snapping shrimp can be found in pairs year-round, and both males and females would benefit from pairing with larger individuals. Larger females are more fecund; males, then, are likely to benefit from pairing with larger females primarily in the reproductive season. Larger individuals are more successful competitors and females benefit more from shared burrow defense than males; for females, then, benefits of pairing with larger males are likely to accrue year-round. In this study, we use field data to test whether within-pair size differences in snapping shrimp correspond more to male or female interests, and whether this outcome differs between seasons. We find that size-assortative pairing varies seasonally: although body sizes of paired males and females are highly correlated year-round, the within-pair size difference is greater during the reproductive season than the nonreproductive season. Furthermore, within pairs, females are larger than males during the reproductive season, while pairs are size-matched or male-biased during the nonreproductive season. These changes in within-pair size relationships suggest seasonal differences in which sex has greater control over pair formation, and highlight nonreproductive benefits associated with monogamous pairing. In addition, these results underscore the importance of considering temporal variation in studies of size-assortative pairing.
Significance statement
In many taxa, it is advantageous for both males and females to mate with larger individuals. As both sexes cannot simultaneously mate with larger individuals, size relationships within pairs reflect the outcome of this sexual conflict. In snapping shrimp, pairs cooperate in defending their burrows from invading conspecifics, and larger individuals are better competitors; larger females are also more fecund. Thus, males obtain a reproductive advantage from mating with larger females, while for females, mating with larger males provides social (territorial defense) benefits. Here, we find seasonal differences in within-pair size relationships, such that females are larger than males during the reproductive season, but pairs in the nonreproductive season are size-matched or male-biased. These results suggest seasonal variation in the outcome of conflict over body size within pairs, and highlights the need to consider temporal variation in size-assortative pairing.



Similar content being viewed by others
Data availability
Data have been archived at https://doi.org/10.6084/m9.figshare.20377113.
Code availability
Code has been archived at https://doi.org/10.6084/m9.figshare.20377125.
References
Baeza JA (2008) Social monogamy in the shrimp Pontonia margarita, a symbiont of Pinctada mazatlanica, off the Pacific coast of Panama. Mar Biol 153:387–395
Baeza JA, Ritson-Williams R, Fuentes MS (2013) Sexual and mating system in a caridean shrimp symbiotic with the winged pearl oyster in the Coral Triangle. J Zool 289:172–181
Baldauf SA, Kullmann H, Schroth SH, Thunken T, Bakker TCM (2009) You can’t always get what you want: size assortative mating by mutual mate choice as a resolution of sexual conflict. BMC Evol Biol 9:129. https://doi.org/10.1186/1471-2148-9-129
Barroso D, Alves DFR, Hirose GL (2019) Testing the resource economic monopolization hypothesis and its consequences for the mating system of Alpheus estuariensis (Decapoda, Caridea, Alpheidae). J Mar Biol Assoc 99:639–647
Bauer RT (2004) Remarkable shrimps. Oklahoma University Press, Norman
Beauchamp G (1999) The evolution of communal roosting in birds: origin and secondary losses. Behav Ecol 10:675–687
Black JM (1996) Introduction: Pair bonds and partnerships. In: Partnerships in birds, the study of monogamy. J. M. Black, editor. New York: Oxford University Press.
Boltaña S, Thiel M (2001) Associations between two species of snapping shrimp, Alpheus inca and Alpheopsis chilensis (Decapoda: Caridea: Alpheidae). J Mar Biol Ass u k 81:633–638
Corey S, Reid DM (1991) Comparative fecundity of decapod crustaceans: I. The fecundity of thirty-three species of nine families of caridean shrimp. Crustaceana 60:270–294
Costa-Souza AC, da Rocha SS, Bezerra LEA, Almeida AO (2014) Breeding and heterosexual pairing in the snapping shrimp Alpheus estuariensis (Caridea: Alpheidae) in a tropical bay in northeastern Brazil. J Crust Biol 34:593–603
Crespi BJ (1989) Causes of assortative mating in arthropods. Anim Behav 38:980–1000
Curran-Everett D (2013) Explorations in statistics: the analysis of ratios and normalized data. Adv Physiol Educ 37:213–219
de Borghezan EA, da Pinto KS, Zuanon J, da Pires THS (2019) Someone like me: size-assortative pairing and mating in an Amazonian fish, sailfin tetra Crenuchus spilurus. PLoS ONE 14:e0222880
Diedenhofen B, Musch J (2015) cocor: a comprehensive solution for the statistical comparison of correlations. PLoS ONE 10(4):e0121945
Diniz P, Rech GS, Ribeiro PHL, Webster MS, Macedo RH (2020) Partners coordinate territorial defense against simulated intruders in a duetting ovenbird. Ecol Evol 10:81–92
Fishelson L (1966) Observations on the littoral fauna of Israel, V. On the habitat and behavior of Alpheus frontalis H. Milne Edwards (Decapoda, Alpheidae). Crustaceana 11:98–104
Fricke HW (1986) Pair swimming and mutual partner guarding in monogamous butterflyfish (Pisces, Chaetodontidae); a joint advertisement for territory. Ethology 73:307–333
Harari AR, Handler AM, Landolt PJ (1999) Size-assortative mating, male choice and female choice in the curculionid beetle Diaprepes abbreviates. Anim Behav 58:1191–1200
Heuring WL (2016) Sexually dimorphic weaponry in monogamous snapping shrimp: investigating the roles of seasonal variation, female aggression, and mate choice. MS thesis, Graduate School of the University of Charleston, Charleston, SC.
Heuring WL, Hughes M (2019) It takes two: seasonal variation in sexually dimorphic weaponry results from divergent changes in males and females. Ecol Evol 9:5433–5439
Heuring WL, Hughes M (2020) Continuously choosy males and seasonally faithful females: sex and season differences underlie size-assortative pairing. Anim Behav 160:91–98
Hoefler CD (2007) Male mate choice and size-assortative pairing in a jumping spider, Phidippus clarus. Anim Behav 73:943–954
Hughes M (1996) Size assessment via a visual signal in snapping shrimp. Behav Ecol Sociobiol 38:51–57
Hughes M, Williamson T, Hollowell K, Vickery R (2014) Sex and weapons: contrasting sexual dimorphisms in weaponry and aggression in snapping shrimp. Ethology 120:982–994
Janicke T, Marie-Orleach L, Aubier TG, Perrier C, Morrow EH (2019) Assortative mating in animals and its role for speciation. Am Nat 194:865–875
Jiang Y, Bolnick DI, Kirkpatrick M (2013) Assortative mating in animals. Am Nat 181:E125–E138
Knowlton N (1980) Sexual selection and dimorphism in two demes of a symbiotic, pair-bonding snapping shrimp. Evolution 34:161–173
Knowlton N, Keller BD (1982) Symmetric fights as a measure of escalation potential in a symbiotic, territorial snapping shrimp. Behav Ecol Sociobiol 10:289–292
Kvarnemo C (2018) Why do some animals mate with one partner rather than many? A review of causes and consequences of monogamy. Biol Rev 93:1795–1812
Linsenmair KE (2007) Sociobiology of terrestrial isopods. In: Duffy JM, Thiel M (eds) Evolutionary ecology of social and sexual systems: crustaceans as model organisms. Oxford University Press, NY, pp 339–364
Luddecke H (2001) Variation in mating pattern in a population of the Andean frog Hyla labialis. Amphibia Reptilia 22:199–207
Mathews LM (2002) Territorial cooperation and social monogamy: factors affecting intersexual behaviours in pair-living snapping shrimp. Anim Behav 63:767–777
Mathews LM (2002) Tests of the mate-guarding hypothesis for social monogamy: does population density, sex ratio, or female synchrony affect behavior of male snapping shrimp (Alpheus angulatus)? Behav Ecol Sociobiol 51:426–432
Mathews LM (2003) Tests of the mate-guarding hypothesis for social monogamy: male snapping shrimp prefer to associate with high-value females. Behav Ecol 14:63–67
Mathews LM (2007) Evidence for high rates of in-pair paternity in the socially monogamous snapping shrimp Alpheus angulosus. Aquat Biol 1:55–62
McLain DK, Boromisa RD (1987) Male choice, fighting ability, assortative mating and the intensity of sexual selection in the milkweed longhorn beetle, Tetraopes tetraophthalmus (Coleoptera, Cerambycidae). Behav Ecol Sociobiol 20:239–246
Moura RR, Gonzaga MO (2017) Temporal variation in size-assortative mating and male mate choice in a spider with ambisexual care. Science of Nature 104:28
Moura RR, Gonzaga MO, Pinto NS, Vasconcellow-Neto J, Requena GS (2021) Assortative mating in time and space: patterns and biases. Ecol Lett 24:1089–1102
Murata Y, Wada K (2002) Population and reproductive biology of an intertidal sandstone-boring isopod, Sphaeroma wadai Nunomura, 1994. J Nat Hist 36:25–35
Nolan BA, Salmon M (1970) The behavior and ecology of snapping shrimp (Crustacea: Alpheus heterochelis and Alpheus normanni). Forma Functio 2:289–335
Parker GA (1983) Mate quality and mating decisions. In Mate Choice (Bateson, P., ed.), pp. 141–166. Cambridge: Cambridge University Press.).
Pavanelli CAM, Mossolin EC, Mantelatto FL (2008) Reproductive strategy of the snapping shrimp Alpheus armillatus H. Milne-Edwards, 1837 in the South Atlantic: fecundity, egg features, and reproductive output. Invertebr Reprod Dev 52:123–130
Pavanelli CAM, Mossolin EC, Mantelatto FL (2010) Maternal investment in egg production: environmental and population-specific effects on offspring performance in the snapping shrimp Alpheus nuttingi (Schmitt, 1924) (Decapoda, Alpheidae). Anim Biol 60:237–247
Pereira A, Tracey E, Cooney PC, Korey CA, Hughes M (2014) Claw regrowth and functional recovery during transformation in the snapping shrimp, Alpheus angulosus. Mar Fresh Behav Physiol 47:147–159
Popp T, Shervette V, Wilber DH (2020) Residency and reproductive patterns of the green porcelain crab, Petrolisthes armatus, on an intertidal oyster reef in its nonnative range. J Exp Mar Biol Ecol 529:151399
R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
Rahman N, Dunham DW, Govind CK (2003) Social monogamy in the big-clawed snapping shrimp, Alpheus heterochelis. Ethology 109:457–473
Reichard UH (2003) Monogamy: past and present. In: Monogamy: mating strategies and partnerships in birds, humans and other mammals. U. H. Reichard and C. Boesch, Ed. New York: Cambridge University Press.
Ridley M (1983) The explanation of organic diversity: the comparative method and adaptations for mating. Clarendon Press, Oxford
Schein H (1975) Aspects of the aggressive and sexual behavior of Alpheus heterochaelis Say. Mar Behav Physiol 3:83–96
Schein H (1977) The role of snapping in Alpheus heterochaelis Say, 1818, the big-clawed snapping shrimp. Crustaceana 33:182–188
Seibt U, Wickler W (1979) The biological significance of the pair-bond in the shrimp Hymenocera picta. Z Tierpsychol 50:166–179
Swenson JE (1993) Hazel grouse (Bonasa bonasia) pairs during the nonbreeding season: mutual benefits of a cooperative alliance. Behav Ecol 4:14–21
Tracey E, Pereira A, Hughes M, Korey CA (2013) The embryonic development of the snapping shrimp, Alpheus angulosus Mcclure, 2002 (Decapoda, Caridea). Crustaceana 86:1367–1381
Wickler W, Seibt U (1981) Monogamy in Crustacea and man. Z Tierpsychol 57:215–234
Wilson, K., and I. C. W. Hardy. 2002. Statistical analysis of sex ratios: an introduction. In: Sex ratios: concepts and research methods, Ed. by I. C. W. Hardy. Cambridge University Press.
Wittenberger JF, Tilson RL (1980) The evolution of monogamy: hypotheses and evidence. Ann Rev Ecol Syst 11:197–232
Acknowledgements
The shrimp used in this research were collected using SC Department of Natural Resources scientific permits #3584 and #3959. We thank the Grice Marine Laboratory and Pete Meier for logistical support.
Funding
This research was supported by the Presidential Summer Research Award in Marine Biology from the Graduate Program in Marine Biology and a Graduate Student Research Grant from the Graduate School at the University of Charleston, both to WH.
Author information
Authors and Affiliations
Contributions
WLH measured the shrimp and performed the statistical analyses. Authors collaborated equally on conception, generating hypotheses, and writing.
Corresponding author
Ethics declarations
Ethics approval
NA.
Conflict of interest
The author declare no competing interests.
Additional information
Communicated by: T. Breithaupt
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heuring, W.L., Hughes, M. It’s the time of the season: seasonal variation in sexually conflicted size-assortative pairing. Behav Ecol Sociobiol 76, 107 (2022). https://doi.org/10.1007/s00265-022-03214-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03214-5