Abstract
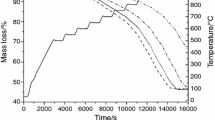
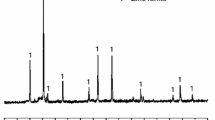
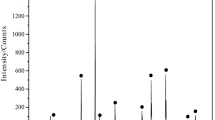
The partial reduction of zinc ferrite to zinc oxide and iron (II, III) oxide can enhance the leaching rate of zinc. Zinc ferrite has often been found as a major component of zinc residues and electric arc furnace dust. The Waelz process is an existing process for zinc ferrite processing, which requires a temperature of more than about 1200 °C to produce Waelz oxide containing ZnO. Since zinc residues have been deemed as a potential way for the extraction of zinc and other critical metals, it is important to analyze the reaction kinetics in the temperature range of interest. This manuscript discusses the chemical kinetics of zinc ferrite reduction by hydrogen gas. Several researchers studied the reduction of zinc ferrite with CO; however, unlike CO, the use of H2 gas as a reductant allows partial reduction of zinc ferrite even at low-temperature range (400–600 °C). Thermogravimetric analyses (TGA) experiments were performed in the temperature range of 300–700 °C with 10% and 30% H2, and the data obtained was analyzed using the isoconversional method of kinetic analyses. The standard models used for isoconversional kinetic analysis were chosen from literature and experimental data were fitted to determine the controlling mechanism and calculation of kinetic parameters of reduction of zinc ferrite. The rate of reaction was found to be increasing with an increase in temperature and partial pressure of H2 gas. The reaction could be divided into three stages, where reaction interface contraction was found to be the controlling mechanism at the initial stage of reaction. As the reaction proceeds, product layer thickness increases, and thus, diffusion was found to be the controlling mechanism. Roasting products obtained from TGA experiments were also characterized using a scanning electron microscope (SEM) to verify critical morphological details.














Similar content being viewed by others
References
Tong LF, Hayes P (2007) Mechanisms of the reduction of zinc ferrites in H2/N2 gas mixtures. Miner Process Extr Metall Rev 28(2):127–157. https://doi.org/10.1080/08827500601012878
Junca E, Grillo FF, Restivo TAG, de Oliveira JR, Espinosa DCR, Tenório JAS (2017) Kinetic investigation of synthetic zinc ferrite reduction by hydrogen. J Therm Anal Calorim 129(2):1215–1223. https://doi.org/10.1007/s10973-017-6222-7
Wu CC, Chang FC, Chen WS, Tsai MS, Wang YN (2014) Reduction behavior of zinc ferrite in EAF-dust recycling with CO gas as a reducing agent. J Environ Manage 143:208–213. https://doi.org/10.1016/j.jenvman.2014.04.005
Junca E, Restivo TAG, de Oliveira JR, Espinosa DCR, Tenório JAS (2016) Reduction of electric arc furnace dust pellets by simulated reformed natural gas. J Therm Anal Calorim 126(3):1889–1897. https://doi.org/10.1007/s10973-016-5629-x
Polsilapa S, Sadedin DR, Wangyao P (2012) Thermodynamics analysis for the zinc ferrite reduction by hydrogen. High Temp Mater Processes (London) 30(6):587–592. https://doi.org/10.1515/htmp.2011.119
Kashyap V, Taylor P (2020) “Selective extraction of zinc from zinc ferrite”, Mining. Metallurgy and Exploration. https://doi.org/10.1007/s42461-020-00306-6
V. Kashyap, P. Taylor, E. Tshijik Karumb, and M. Cheshire, “Application of zinc ferrite reduction in the extraction of Zn, Ga and In from zinc refinery residue,” Minerals Engineering, vol. 171, no. July, p. 107078, 2021, https://doi.org/10.1016/j.mineng.2021.107078.
V. Kashyap and P. Taylor, “Extraction and recovery of zinc and indium from residue rich in zinc ferrite,” Minerals Engineering, vol. 176, no. December 2021, p. 107364, 2022, https://doi.org/10.1016/j.mineng.2021.107364.
N. Peng et al., “Thermochimica Acta Isothermal kinetics of zinc ferrite reduction using a CO-CO 2 -Ar gas mixture,” vol. 686, no. August 2019, 2020, https://doi.org/10.1016/j.tca.2020.178564.
Wang X, Yang D, Ju S, Peng J, Duan X (2013) Thermodynamics and kinetics of carbothermal reduction of zinc ferrite by microwave heating. Transactions of Nonferrous Metals Society of China 23(12):3808–3815. https://doi.org/10.1016/S1003-6326(13)62933-7
G. Wang, X. Min, N. Peng, and Z. Wang, “The isothermal kinetics of zinc ferrite reduction with carbon monoxide,” Journal of Thermal Analysis and Calorimetry, no. December 2020, 2021, https://doi.org/10.1007/s10973-020-10542-z.
Junca E, De Oliveira JR, Restivo TAG, Espinosa DCR, Tenório JAS (2016) Synthetic zinc ferrite reduction by means of mixtures containing hydrogen and carbon monoxide. J Therm Anal Calorim 123(1):631–641. https://doi.org/10.1007/s10973-015-4973-6
Khawam A, Flanagan DR (2006) Solid-state kinetic models : basics and mathematical fundamentals solid-state kinetic models : basics and mathematical fundamentals. J Phys Chem 110:17315–17328. https://doi.org/10.1021/jp062746a
S. Sarbishei and L. T. Tafaghodi Khajavi, “Kinetic analysis on nickel laterite ore calcination using model-free and model-fitting methods,” Minerals Engineering, vol. 136, no. March, pp. 129–139, 2019, https://doi.org/10.1016/j.mineng.2019.03.010.
M. Ramezani, P. Tremain, E. Doroodchi, and B. Moghtaderi, “Determination of carbonation/calcination reaction kinetics of a limestone sorbent in low CO2 partial pressures using TGA experiments,” Energy Procedia, vol. 114, no. November 2016, pp. 259–270, 2017, https://doi.org/10.1016/j.egypro.2017.03.1168.
Yu D, Zhu M, Utigard TA, Barati M (2013) TGA kinetic study on the hydrogen reduction of an iron nickel oxide. Miner Eng 54:32–38. https://doi.org/10.1016/j.mineng.2013.03.018
Barde AA, Klausner JF, Mei R (2016) Solid state reaction kinetics of iron oxide reduction using hydrogen as a reducing agent. Int J Hydrogen Energy 41(24):10103–10119. https://doi.org/10.1016/j.ijhydene.2015.12.129
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520(1–2):1–19. https://doi.org/10.1016/j.tca.2011.03.034
Nouri SMM, Ebrahim HA, Jamshidi E (2011) Simulation of direct reduction reactor by the grain model. Chem Eng J 166(2):704–709. https://doi.org/10.1016/j.cej.2010.11.025
M. G. Gustum, “Modelling of gas-solid reactions,” no. June, 2018.
L. F. Tong, “The mechanisms and kinetics of reduction of zinc oxide solid solutions,” Mineral Processing and Extractive Metallurgy Review, vol. 23, no. January 2015, pp. 11–50, 2010, https://doi.org/10.1080/08827500214515.
A. Pineau, N. Kanari, and I. Gaballah, “Kinetics of reduction of iron oxides by H2 Part 1: low temperature reduction of hematite,” Thermochimica Acta, vol. 456, no. 2, pp. 75–88, 2005, .1037//0033–2909.I26.1.78.
Acknowledgements
The authors wish to gratefully acknowledge the Critical Materials Institute (CMI) for supporting this work under Focus Area CMI 1.1.13 (Recovery of Critical Materials as By-Products). The authors wish to acknowledge Dr. Michael Sanders at the Colorado School of Mines for his diligent experimental work.
This study is a presentation of our own work. The authors declare that there is no potential conflict of interest. This work was supported by the Critical Materials Institute, an Energy Innovation Hub funded by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office. The experimental work was carried out by Dr. Michael Sanders at the Colorado School of Mines.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vivek Kashyap’s current affiliation: Brimstone Energy, Oakland, CA, USA
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kashyap, V., Karumb, E.T. & Taylor, P. TGA Kinetic Analyses of Zinc Ferrite Reduction with H2. Mining, Metallurgy & Exploration 39, 2167–2178 (2022). https://doi.org/10.1007/s42461-022-00661-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-022-00661-6