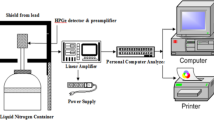
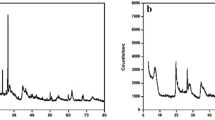
Abstract
Adsorption behaviour of 95Zr(IV), 181Hf(IV) and 95Nb(V) radionuclides onto bentonite is investigated in this study. Optimization of parameters is governed by conducting the adsorption experiments at different pHs, contact time, adsorbent mass and temperature. Distribution coefficient values of 21,388, 19,869 and 12,866 mL/g for 95Zr(IV), 181Hf(IV) and 95Nb(V) radionuclides, respectively, are achieved at pH 2.5. Contact time of 120 min was sufficient to reach equilibrium and the Lagergren kinetic model was appropriate to describe the kinetic data of zirconium. Of the studied adsorption isotherms, Redlich–Peterson was the best model to fit the adsorption equilibrium data of zirconium onto bentonite. The maximum adsorption capacity of bentonite toward zirconium is found to be 63.35 mg/g. The obtained values of enthalpy change and free energy change showed that adsorption of radionuclides onto bentonite was endothermic and spontaneous processes. Bentonite succeeded to efficiently remove the studied radionuclides even in presence of high concentrations of foreign ions. Bentonite succeeded to adsorb 95Zr(IV), 181Hf(IV) and 95Nb(V) radionuclides from radioactive process wastewater with distribution coefficient values of 10,422, 15,453 and 9774 mL/g, respectively.












Similar content being viewed by others
References
Yavari R, Davarkhah R (2013) Application of modified multiwall carbon nanotubes as a sorbent for zirconium (IV) adsorption from aqueous solution. J Radioanal Nucl Chem 298:835–845. https://doi.org/10.1007/s10967-013-2476-0
Bhatti HN, Zaman Q, Kausar A, Noreen S, Iqbal M (2016) Efficient remediation of Zr(IV) using citrus peel waste biomass: kinetic, equilibrium and thermodynamic studies. Ecol Eng 95:216–228. https://doi.org/10.1016/j.ecoleng.2016.06.087
Hanif A, Bhatti HN, Hanif MF (2015) Removal of zirconium from aqueous solution by Ganoderma lucidum: biosorption and bioremediation studies. Desalin Water Treat 53:195–205. https://doi.org/10.1080/19443994.2013.837005
Akhtar K, Akhtar MW, Khalid AM (2008) Removal and recovery of zirconium from its aqueous solution by Candida tropicalis. J Hazard Mater 156:108–117. https://doi.org/10.1016/j.jhazmat.2007.12.002
Faghihian H, Kabiri-Tadi M (2010) Removal of zirconium from aqueous solution by modified clinoptilolite. J Hazard Mater 178:66–73. https://doi.org/10.1016/j.jhazmat.2010.01.044
Varala S, Dharanija B, Satyavathi B, Rao VVB, Parthasarathy R (2016) New biosorbent based on deoiled karanja seed cake in biosorption studies of Zr(IV): optimization using box-behnken method in response surface methodology with desirability approach. Chem Eng J 302:786–800. https://doi.org/10.1016/j.cej.2016.05.088
Smolik M, Jakóbik-Kolon A, Porański M (2009) Separation of zirconium and hafnium using Diphonix® chelating ion-exchange resin. Hydrometallurgy 95:350–353. https://doi.org/10.1016/j.hydromet.2008.05.010
Hasany SM, Shamsi AM, Rauf MA (1997) Sorption of hafnium on hydrous titanium oxide using radiotracer technique. J Radioanal Nucl Chem 219:51–54. https://doi.org/10.1007/bf02040264
Grayson M, Othmer K (1980) Encyclopedia of chemical technology. Wiley, New York, p 73
Hampel CA, Hawley GG (1973) The encyclopedia of chemistry. Van Nostrand Reinhold, New York, p 515
De AK, Khopkar SM, Chalmers RA (1970) Solvent extraction of metals. Van Nostrand Reinhold, London, pp 241–242
Samaddar P, Sen K (2014) Cloud point extraction: a sustainable method of elemental preconcentration and speciation. J Indu Eng Chem 20:1209–1219. https://doi.org/10.1016/j.jiec.2013.10.033
Kurniawan TA, Chan GYS, Lo W, Babel S (2006) Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118:83–98. https://doi.org/10.1016/j.cej.2006.01.015
Rubio J, Souza ML, Smith RW (2002) Overview of flotation as a wastewater treatment technique. Miner Eng 15:139–155
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Mahmoud MR, Rashad GM, Elewa AM, Metwally E, Saad EA (2019) Optimization of adsorption parameters for removal of 152+154Eu(III) from aqueous solutions by using Zn-Cu-Ni ternary mixed oxide. J Mol Liq 291:111257. https://doi.org/10.1016/j.molliq.2019.111257
Rashad GM, Mahmoud MR, Elewa AM, Metwally E (2016) Saad EA (2016) Removal of radiocobalt from aqueous solutions by adsorption onto low-cost adsorbents. J Radioanal Nucl Chem 309:1065–1076
Soliman MA, Rashad GH, Mahmoud MR (2019) Organo-modification of montmorillonite for enhancing the adsorption efficiency of cobalt radionuclides from aqueous solutions. Environ Sci Pollut Res 26:10398–10413. https://doi.org/10.1007/s11356-019-04478-7
Mahmoud MR, Rashad GH, Metwally E, Saad EA, Elewa AM (2017) Adsorptive removal of 134Cs+, 60Co2+ and 152+154Eu3+ radionuclides from aqueous solutions using sepiolite: Single and multi-component systems. Appl Clay Sci 141:72–80. https://doi.org/10.1016/j.clay.2016.12.021
Hassan RS, Abass MR, Eid MA, Abdel-Galil EA (2021) Sorption of some radionuclides from liquid waste solutions using anionic clay hydrotalcite sorbent. Appl Radiat Isot 178:109985. https://doi.org/10.1016/j.apradiso.2021.109985
Yu T, Xu Z, Ye J (2019) Adsorption kinetics of Eu(III) and Am(III) onto bentonite: analysis and application of the liquid membrane tidal diffusion model. J Radioanal Nuclear Chem 319:749–757. https://doi.org/10.1007/s10967-018-6386-z
Seliman AF, Lasheen YF, Youssief MAE, Abo-Aly MM, Shehata FA (2014) Removal of some radionuclides from contaminated solution using natural clay: bentonite. J Radioanal Nucl Chem 300:969–979. https://doi.org/10.1007/s10967-014-3027-z
Liu C, Xu Q, Xu Y, Wang B, Long H, Fang S, Zhou D (2022) Characterization of adsorption behaviors of U(VI) on bentonite colloids: batch experiments, kinetic evaluation and thermodynamic analysis. J Radioanal Nucl Chem 331:597–607. https://doi.org/10.1007/s10967-021-08123-x
Khan SA (2003) Sorption of the long-lived radionuclides cesium-134, strontium-85 and cobalt-60 on bentonite. J Radioanal Nucl Chem 258:3–6
Attar LA, Safia B, Abdul Ghani B (2021) Adsorption behaviour of 226Ra and 210Pb onto thermally treated forms of bentonite. J Radioanal Nucl Chem 327:1167–1178. https://doi.org/10.1007/s10967-021-07606-1
Amayri S, Fröhlich DR, Kaplan U, Trautmann N, Reich T (2016) Distribution coefficients for the sorption of Th, U, Np, Pu, and Am on Opalinus Clay. Radiochim Acta 104:33–40. https://doi.org/10.1515/ract-2015-2409
Zhang J, Mallants D, Brady PV (2022) Molecular dynamics study of uranyl adsorption from aqueous solution to smectite. Appl Clay Sci 218:106361. https://doi.org/10.1016/j.clay.2021.106361
Guerra DJL, Menonca ES, Silva RAR, Lara W (2012) Thermodynamic, equilibrium and kinetic studies of adsorption of pillarized and organofunctionalized smectite clay for Th4+ removal. J Ceram Sci Tech 3:17–28
Hasany SM, Chaudhary MH (1989) Adsorption of traces of hafnium on manganese dioxide from acid solutions. J Radioanal Nucl Chem 131:425–434
Ghosh M, Devi PSR, Verma R, Reddy AVR (2015) Sorption of niobium on colloidal silica and the effect of humic acid. J Radioanal Nucl Chem 306:147–153. https://doi.org/10.1007/s10967-015-4055-z
Söderlund M, Hakanen M, Lehto J (2015) Sorption of niobium on boreal forest soil. Radiochim Acta 103:859–869. https://doi.org/10.1515/ract-2015-2429
Yamaguchi T, Ohira S, Hemmi K, Barr L, Shimada A, Maeda T, Iida Y (2020) Consideration on modeling of Nb sorption onto clay minerals. Radiochim Acta 108:873–877. https://doi.org/10.1515/ract-2020-0006
Dasa A, Chandrakumar KRS, Paul B, Chopade SM, Majumdar S, Singh AK, Kain V (2020) Enhanced adsorption and separation of zirconium and hafnium under mild conditions by phosphoric acid based ligand functionalized silica gels: insights from experimental and theoretical investigations. Sep Purif Technol 239:116518. https://doi.org/10.1016/j.seppur.2020.116518
Takamiya K, Fukunishi T, Tsujito R, Fukutani S, Takahashi T, Shibata S, Uchida S (2007) Adsorption of fission products onto soils using a fission multitracer. J Radioanal Nucl Chem 273:195–198. https://doi.org/10.1007/s10967-007-0735-7
Kubica B, Tuteja-Krysa M, Godunowa H, Szeglowski Z (2001) Adsorption of Hf and Nb on copper and zinc hexacyanoferrate(II) from HCl and H2SO4 solutions. J Radioanal Nucl Chem 247(535):539
Dyer A, Kadhim FH (1989) Inorganic ion-exchangers for the removal of zirconium, hafnium and niobium radioisotopes from aqueous solutions. J Radioanal Nucl Chem 131:161–169
Monroy-Guzman F, Trubert D, Le Naour C (2002) Adsorption behavior of Zr, Hf, Nb, Ta and Pa on macroporous anion exchanger in NH4SCN/HClO4 and NH4SCN/HF media. J Radioanal Nucl Chem 254:431–437
El-Sweify FH, El-Shazly EAA, Salama SM (2018) Comparison of some organic and inorganic ion exchangers concerning the sorption of Ce(III), Te(IV), Zr(IV), Hf(IV) and Nb(V). Radiochim Acta 106:207–2216. https://doi.org/10.1515/ract-2017-2789
Sari A, Tuzen M (2014) Cd(II) adsorption from aqueous solution by raw and modified kaolinite. Appl Clay Sci 88–89:63–72. https://doi.org/10.1016/j.clay.2013.12.021
Zhang H, Xu M, Wanga H, Lei D, Qu D, Zhai Y (2013) Adsorption of copper by aminopropyl functionalized mesoporous delta manganese dioxide from aqueous solution. Colloids Surf A Physicochem Eng Aspects 435:78–84. https://doi.org/10.1016/j.colsurfa.2012.12.037
Chen JJ, Ahmad AL, Ooi BS (2013) Poly(N-isopropylacrylamide-co-acrylic acid) hydrogels for copper ion adsorption: equilibrium isotherms, kinetic and thermodynamic studies. J Environl Chem Eng 1:339–348. https://doi.org/10.1016/j.jece.2013.05.012
An F, Gao B, Dai X, Wang M, Wang X (2011) Efficient removal of heavy metal ions from aqueous solution using salicylic acid type chelate adsorbent. J Hazard Mater 192:956–962. https://doi.org/10.1016/j.jhazmat.2011.05.050
Caliskan N, Kul AR, Alkan S, Sogut EG, Alacabey I (2011) Adsorption of Zinc(II) on diatomite and manganese-oxide-modified diatomite: a kinetic and equilibrium study. J Hazard Mater 193:27–36. https://doi.org/10.1016/j.jhazmat.2011.06.058
Shakir K, Ghoneimy HF, Hennawy IT, Elkafrawy A, Beheir SG, Refaat M (2011) Simultaneous removal of chromotrope 2B and radionuclides from mixed radioactive process wastewater using organo-bentonite. Eur J Chem 2:83–93
Wu P, Zhang Q, Dai Y, Zhu N, Dang Z, Li P, Wu J, Wang X (2011) Adsorption of Cu(II), Cd(II) and Cr(III) ions from aqueous solutions on humic acid modified Ca-montmorillonite. Geoderma 164:215–219. https://doi.org/10.1016/j.geoderma.2011.06.012
Bhatti HN, Amin M (2013) Removal of zirconium(IV) from aqueous solution by Coriolus versicolor: equilibrium and thermodynamic study. Ecol Eng 51:178–180. https://doi.org/10.1016/j.ecoleng.2012.12.078
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Suenska Vetenskapsakademiens Handlingar 24:1–39
Ritchie AG (1977) Alternative to the Elovich equation for the kinetics of adsorption of gases on solids. J Chem Soc Faraday Trans 1(73):1650–1653. https://doi.org/10.1039/F19777301650
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–60
Mahmoud MR, Seliman AF (2014) Evaluation of silica/ferrocyanide composite as a dual-function material for simultaneous removal of 137Cs+ and 99TcO4- from aqueous solutions. Appl Radiat Isot 91:141–154. https://doi.org/10.1016/j.apradiso.2014.05.021
Mahmoud MR, Soliman MA, Allan KF (2015) Adsorption behavior of samarium(III) from aqueous solutions onto PAN@SDS core-shell polymeric adsorbent. Radiochim Acta 103:443–456. https://doi.org/10.1515/ract-2014-2299
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Temkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim USSR 12:327–356
Redlich O, Peterson DLA (1959) Useful adsorption isotherm. J Phys Chem 63:1024–1026
Hamoud MA, Allan KF, Ayoub RR, Holeil M, Mahmoud MR (2021) Efficient removal of radiocobalt and manganese from their binary aqueous solutions by batch adsorption process using PAN/HDTMA/KCuHCF composite. Radiochim Acta 109:27–39. https://doi.org/10.1515/ract-2020-0078
Petrucci RH, Harwood WS (1997) General chemistry: principles and modern applications, 7th edn. Prentice Hall, New Jersey, p 711
Mahmoud MR, Lazaridis NK (2015) Simultaneous removal of nickel(II) and chromium(VI) from aqueous solutions and simulated wastewaters by foam separation. Sep Sci Technol 50:1421–1432. https://doi.org/10.1080/01496395.2014.978456
Mahmoud MR, Someda HH (2012) Mg–Al layered double hydroxide intercalated with sodium lauryl sulfate as a sorbent for 152+154Eu from aqueous solutions. J Radioanal Nucl Chem 292:1391–1400. https://doi.org/10.1007/s10967-012-1715-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All of the authors consent to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mekawy, Z.A., El Shazly, E.A.A. & Mahmoud, M.R. Utilization of bentonite as a low-cost adsorbent for removal of 95Zr(IV), 181Hf(IV) and 95Nb(V) radionuclides from aqueous solutions. J Radioanal Nucl Chem 331, 3935–3948 (2022). https://doi.org/10.1007/s10967-022-08432-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08432-9