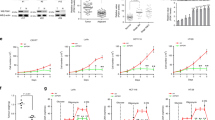
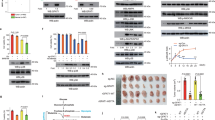
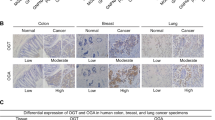
Abstract
Oncogenic Kras-activated pancreatic ductal adenocarcinoma (PDAC) cells highly rely on an unconventional glutamine catabolic pathway to sustain cell growth. However, little is known about how this pathway is regulated. Here we demonstrate that Kras mutation induces cellular O-linked β-N-acetylglucosamine (O-GlcNAc), a prevalent form of protein glycosylation. Malate dehydrogenase 1 (MDH1), a key enzyme in the glutamine catabolic pathway, is positively regulated by O-GlcNAcylation on serine 189 (S189). Molecular dynamics simulations suggest that S189 glycosylation on monomeric MDH1 enhances the stability of the substrate-binding pocket and strengthens the substrate interactions by serving as a molecular glue. Depletion of O-GlcNAcylation reduces MDH1 activity, impairs glutamine metabolism, sensitizes PDAC cells to oxidative stress, decreases cell proliferation and inhibits tumor growth in nude mice. Furthermore, O-GlcNAcylation levels of MDH1 are elevated in clinical PDAC samples. Our study reveals that O-GlcNAcylation contributes to pancreatic cancer growth by regulating the metabolic activity of MDH1.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The human pancreatic tumor data (Fig. 1c) were derived from the TCGA Research Network (http://cancergenome.nih.gov). The crystal structure of a ternary complex of porcine cytoplasmic malate dehydrogenase, α-ketomalonate and tetrahydro-NAD was obtained from the PDB database (https://www.rcsb.org/structure/5MDH). The crystal structure of hMDH1 is available at https://www.rcsb.org/structure/7RM9. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Zhang, J., Pavlova, N. N. & Thompson, C. B. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 36, 1302–1315 (2017).
Michalak, K. P., Maćkowska-Kędziora, A., Sobolewski, B. & Woźniak, P. Key roles of glutamine pathways in reprogramming the cancer metabolism. Oxid. Med. Cell. Longevity 2015, 964321 (2015).
Anastasiou, D. & Cantley, L. C. Breathless cancer cells get fat on glutamine. Cell Res. 22, 443–446 (2012).
Xiang, Y. et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J. Clin. Invest. 125, 2293–2306 (2015).
Csibi, A. et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 153, 840–854 (2013).
Yang, W.-H., Qiu, Y., Stamatatos, O., Janowitz, T. & Lukey, M. J. Enhancing the efficacy of glutamine metabolism inhibitors in cancer therapy. Trends Cancer 7, 790–804 (2021).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Yang, S. et al. Mitochondrial glutamine metabolism via GOT2 supports pancreatic cancer growth through senescence inhibition. Cell Death Dis. 9, 55 (2018).
Tong, Y. et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol. Cell 81, 2303–2316 (2021).
Wang, Y.-P. et al. Arginine methylation of MDH1 by CARM1 inhibits glutamine metabolism and suppresses pancreatic cancer. Mol. Cell 64, 673–687 (2016).
Torres, C. R. & Hart, G. W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 259, 3308–3317 (1984).
Parker, M. P., Peterson, K. R. & Slawson, C. O-GlcNAcylation and O-GlcNAc cycling regulate gene transcription: emerging roles in cancer. Cancers 13, 1666 (2021).
Li, X. et al. O-GlcNAcylation of core components of the translation initiation machinery regulates protein synthesis. Proc. Natl Acad. Sci. USA 116, 7857–7866 (2019).
Zhu, Q. et al. O-GlcNAcylation regulates the methionine cycle to promote pluripotency of stem cells. Proc. Natl Acad. Sci. USA 117, 7755–7763 (2020).
Wang, Y. et al. O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc. Natl Acad. Sci. USA 114, 13732–13737 (2017).
Slawson, C. & Hart, G. W. O-GlcNAc signalling: implications for cancer cell biology. Nat. Rev. Cancer 11, 678–684 (2011).
Peterson, S. B. & Hart, G. W. New insights: a role for O-GlcNAcylation in diabetic complications. Crit. Rev. Biochem. Mol. Biol. 51, 150–161 (2016).
Wani, W. Y., Chatham, J. C., Darley-Usmar, V., McMahon, L. L. & Zhang, J. O-GlcNAcylation and neurodegeneration. Brain Res. Bull. 133, 80–87 (2017).
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Bryant, K. L., Mancias, J. D., Kimmelman, A. C. & Der, C. J. KRAS: feeding pancreatic cancer proliferation. Trends Biochem. Sci. 39, 91–100 (2014).
Santana-Codina, N. et al. Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis. Nat. Commun. 9, 4945 (2018).
Ricciardiello, F. et al. Suppression of the HBP function increases pancreatic cancer cell sensitivity to a pan-RAS inhibitor. Cells 10, 431 (2021).
Qian, K. et al. Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer. J. Biol. Chem. 293, 13989–14000 (2018).
Clark, P. M. et al. Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. J. Am. Chem. Soc. 130, 11576–11577 (2008).
Ding, N. et al. Thiamet-G-mediated inhibition of O-GlcNAcase sensitizes human leukemia cells to microtubule-stabilizing agent paclitaxel. Biochem. Biophys. Res. Commun. 453, 392–397 (2014).
Martin, S. E. S. et al. Structure-based evolution of low nanomolar O-GlcNAc transferase inhibitors. J. Am. Chem. Soc. 140, 13542–13545 (2018).
Rexach, J. E. et al. Quantification of O-glycosylation stoichiometry and dynamics using resolvable mass tags. Nat. Chem. Biol. 6, 645–651 (2010).
Gao, H. et al. A widely compatible expression system for the production of highly O-GlcNAcylated recombinant protein in Escherichia coli. Glycobiology 28, 949–957 (2018).
Breiter, D. R., Resnik, E. & Banaszak, L. J. Engineering the quaternary structure of an enzyme: construction and analysis of a monomeric form of malate dehydrogenase from Escherichia coli. Protein Sci. 3, 2023–2032 (1994).
Chapman, A. D. M., Cortés, A., Dafforn, T. R., Clarke, A. R. & Brady, R. L. Structural basis of substrate specificity in malate dehydrogenases: crystal structure of a ternary complex of porcine cytoplasmic malate dehydrogenase, α-ketomalonate and tetrahydro-NAD. J. Mol. Biol. 285, 703–712 (1999).
McCue, W. M. & Finzel, B. C. Structural characterization of the human cytosolic malate dehydrogenase I. ACS Omega 7, 207–214 (2022).
Lee, J. et al. CHARMM-GUI supports the Amber force fields. J. Chem. Phys. 153, 035103 (2020).
Sharma, N. K., Sethy, N. K. & Bhargava, K. Comparative proteome analysis reveals differential regulation of glycolytic and antioxidant enzymes in cortex and hippocampus exposed to short-term hypobaric hypoxia. J. Proteomics 79, 277–298 (2013).
Alkan, H. F. et al. Cytosolic aspartate availability determines cell survival when glutamine is limiting. Cell Metab. 28, 706–720 (2018).
Levine, Z. G. et al. Mammalian cell proliferation requires noncatalytic functions of O-GlcNAc transferase. Proc. Natl Acad. Sci. USA 118, e2016778118 (2021).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Kerk, S. A., Papagiannakopoulos, T., Shah, Y. M. & Lyssiotis, C. A. Metabolic networks in mutant KRAS-driven tumours: tissue specificities and the microenvironment. Nat. Rev. Cancer 21, 510–525 (2021).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signaling 6, pl1 (2013).
Nie, H. et al. O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth. Nat. Commun. 11, 36 (2020).
Kumar, R., Iyer, V. G. & Im, W. CHARMM-GUI: a graphical user interface for the CHARMM users. In Abstracts of papers of the American Chemical Society 233, 273 (American Chemical Society, 2007).
The PyMOL Molecular Graphics System v.1.8 (Schrödinger, LLC, 2015).
Sanner, M. F. Python: a programming language for software integration and development. J. Mol. Graphics Modell. 17, 57–61 (1999).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089 (1993).
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFF1200404 and 2021YFA1201201 to R.Z., 2017YFA0505002 to W.Q.), the National Science Foundation of China (NSFC, grant nos. 31971212 and 91753125 to W.Yi., U1967217 to R.Z.), the National Science Foundation of Zhejiang Province (LZ21C050001 to W.Yi.), the Mizutani Foundation for Glycoscience (210036 to W.Yi.), National Independent Innovation Demonstration Zone Shanghai Zhangjiang Major Projects (ZJZX2020014), Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (SN-ZJU-SIAS-003), Fundamental Research Funds for Central Universities (226-2022-00043, 226-2022-00192 and K20220228) and BirenTech Research (BR-ZJU-SIAS-001). Y.W. acknowledges the access to computational resources from the Information Technology Center and State Key Lab of Computer-Aided Design (CAD) & Computer Graphics (CG) of Zhejiang University. R.Z. also acknowledges financial support from the W. M. Keck Foundation (grant award 2019–2022).
Author information
Authors and Affiliations
Contributions
W.Yi. conceived the project; W.Yi. designed the cell biology and biochemistry experiments; R.Z. designed the molecular dynamics simulation experiments; Q.Z., D.G., W. Yang and Z.L. performed the cell biology, biochemistry and xenograft experiments; H.Z. and Y.W. performed the molecular dynamics simulation experiments; Z.F. and W.Q. performed the mass spectrometry analysis; L.W., Y.W., W.Q., R.Z. and W.Yi. analyzed the data; L.W. and J.Z. provided tumor tissue samples; Q.Z. and W.Yi. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Matthew Pratt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 OGT depletion inhibits cell proliferation in PDAC cells.
a, Quantification and statistical analysis of OGT and O-GlcNAcylation levels from 24 pairs of human pancreatic tumor (T) and adjacent peritumoral (N) tissue samples. b, Immunoblotting analysis of OGT and O-GlcNAcylation levels in pancreas ductal epithelial cells (HPDE) and PDAC cells (AsPC-1, HPAC, HPAF-II, 8988 T, SW1990 and PANC1). c and d, Immunoblotting of OGT levels in HPDE and PDAC cells stably transfected with scramble or shOGT. e, Relative cell proliferation rate of PDAC cells stably transfected with scramble or shOGT (n = 3 independent assays). Immunoblots are representative of three independent experiments. Error bars of data in e denote the means ± SD. Statistical analyses were performed by two-tailed Student’s t-test.
Extended Data Fig. 2 Kras activated mutation promotes OGT expression in PDAC cells.
a, Immunoblotting of GFPT, OGT and O-GlcNAc levels in HPDE cells upon ectopic expression of KrasG12D. b, Immunoblotting of O-GlcNAc levels in SW1990 cells upon depletion of Kras. c, Immunoblotting of O-GlcNAc levels upon OGT depletion in HPDE cells expressing KrasG12D. d, Cell proliferation rate of HPDE cells expressing KrasG12D upon OGT depletion (n = 3 independent assays). Immunoblots are representative of three independent experiments. Error bars of data in d denote the means ± SD. Statistical analyses were performed by two-tailed Student’s t-test.
Extended Data Fig. 3 Analysis of relative abundance of metabolites upon OGT depletion.
a–b, Relative abundance of metabolites in the TCA cycle (a) and the serine metabolism (b) in SW1990 cells stably transfected with scramble or shOGT (n = 3 independent assays). c–e, Relative abundance of metabolites in the Gln metabolism (c), the TCA cycle (d) and the serine metabolism (e) in HPDE cells stably transfected with scramble or shOGT (n = 3 independent assays). Error bars of data in a, b, c, d and e denote the means ± SD. Statistical analyses were performed by two-tailed Student’s t-test.
Extended Data Fig. 4 Key enzymes in the Gln metabolism are O-GlcNAc modified.
a–c, Analysis of O-GlcNAcylation levels of GOT1 (a), MDH1 (b) and ME1 (c) in the presence of TMG (100 μM) or OGT overexpression in HEK293T cells. d–f, Analysis of O-GlcNAcylation levels of GOT1 (d), MDH1 (e) and ME1 (f) in the presence of OSMI-4 (20 μM) in HEK293T cells. Immunoblots are representative of three independent experiments.
Extended Data Fig. 5 Molecular dynamics simulations of substrate-bound MDH1.
a, The definition of NADH-binding site. Eight residues, shown in stick and colored in magenta, around NADH were selected to represent the NADH’s binding site. b, Time-dependent COM distance between NADH and binding site of the nonglycosylated (left) and glycosylated S189 forms (right) in pMDH1. The thin lines represent the trajectories of each simulation while the thick line represents the average. c, The distribution of COM distance between NADH and its binding site. d, The definition of MAK-binding site. Five residues, shown in stick and colored in magenta, around MAK were selected to represent the MAK’s binding site. e, Time-dependent COM distance between MAK and binding site of the nonglycosylated (left) and glycosylated S189 forms (right). f, The distribution of COM distance between MAK and its binding site.
Extended Data Fig. 6 Glycosylation stabilizes substrate-enzyme interactions.
a, The residue root-mean-square fluctuation (RMSF) of pMDH1. The difference is the RMSF value of nonglycosylated simulations minus the RMSF value of the glycosylated simulations. A positive value indicates a residue is more rigid with S189 glycosylation while a negative value indicates a residue is more flexible. b, Comparison of the interaction network (contact probability) between MDH1 with NADH (left), and MDH1 with MAK (right) of WT (top) and MDH1 with glycosylated S189 (bottom). Only the residues with a probability of more than 10% are shown. c, The overall average contact probabilities between NADH and MAK of WT (left) MDH1 with glycosylated S189 (right). d, The snapshot of the interaction network between substrates and glycosylated S189. e, The contact probability between GlcNAc and all residues of MDH1, and a schematic diagram to show the major contact residues. f, Relative enzymatic activity of WT or the triplet mutant of MDH1 in the presence or absence of OGT overexpression (n = 5 independent assays). Error bars of data in f denote the means ± SD. Statistical analyses were performed by two-tailed Student’s t-test.
Extended Data Fig. 7 MDH1 S189 glycosylation promotes PDAC cell proliferation.
a, Immunoblotting of MDH1 expression in HPDE and PDAC cells upon stably transfection with scramble or shMDH1. b, Relative cell proliferation rate of HPDE and PDAC cells upon stably transfection with scramble or shMDH1 (n = 3 independent assays). c, Immunoblotting of MDH1 in PANC-1 cells stably expressing scramble shRNA, shMDH1, shRNA-resistant WT or S189A MDH1. d and e, Cell proliferation rate of SW1990 (c) and PANC-1 cells (d) stably expressing scramble shRNA, shMDH1, shRNA-resistant WT or S189A MDH1 (n = 5 independent assays). f and g, Relative clone numbers generated from SW1990 (f) and PANC-1 cells (g) stably expressing scramble shRNA, shMDH1, shRNA-resistant WT or S189A MDH1 (n = 5 independent assays). h and i, Cell proliferation rate of SW1990 (h) and PANC-1 cells (i) reconstituted with WT or S189A MDH1 upon OGT overexpression (n = 5 independent assays). Immunoblots are representative of three independent experiments. Error bars of data in b, d, e, f, g, h and i denote the means ± SD. Statistical analyses were performed by two-tailed Student’s t-test.
Extended Data Fig. 8 MDH1 glycosylation promotes PDAC cell proliferation in part through redox regulation.
a, Relative cell proliferation of SW1990 cells reconstituted with WT or S189A MDH1, in the presence or absence of ME1 depletion and malate (n = 5 independent assays). b, Immunoblotting of SW1990 cells reconstituted with WT or S189A MDH1, in the presence or absence of ME1 depletion and malate. c, Relative cell number of SW1990 cells reconstituted with WT or S189A MDH1 after treatment with 5 mM NAC, 5 mM GSH or 4 mM cell-permeable malate for 24 h (n = 5 independent assays). d, Cell survival rate of SW1990 cells reconstituted with WT or S189A MDH1 after treatment with indicated concentrations of H2O2 for 1 h (n = 5 independent assays). e, Relative cell number of SW1990 cells reconstituted with WT or S189A MDH1 after incubation with 4 mM cell-permeable malate for 12 h, followed by treatment with 0.3 mM H2O2 for 16 h (n = 5 independent assays). Error bars of data in a, c, d and e denote the means ± SD. Statistical analyses were performed by two-tailed Student’s t-test.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Supplementary Figs. 1–16 and unmodified western blots of supplementary figures.
Supplementary Video 1
Molecular dynamics simulation of glycosylated MDH1.
Supplementary Video 2
Molecular dynamics simulation of nonglycosylated MDH1.
Supplementary Data 1
Supplementary statistical source data.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Zhu, Q., Zhou, H., Wu, L. et al. O-GlcNAcylation promotes pancreatic tumor growth by regulating malate dehydrogenase 1. Nat Chem Biol 18, 1087–1095 (2022). https://doi.org/10.1038/s41589-022-01085-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-01085-5
This article is cited by
-
A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle
Journal of Experimental & Clinical Cancer Research (2024)
-
Direct stimulation of de novo nucleotide synthesis by O-GlcNAcylation
Nature Chemical Biology (2024)
-
The Deinococcus protease PprI senses DNA damage by directly interacting with single-stranded DNA
Nature Communications (2024)
-
O-GlcNAcylation of E3 ubiquitin ligase SKP2 promotes hepatocellular carcinoma proliferation
Oncogene (2024)
-
The GFPT2-O-GlcNAcylation-YBX1 axis promotes IL-18 secretion to regulate the tumor immune microenvironment in pancreatic cancer
Cell Death & Disease (2024)