Abstract
Purpose
Integrins αv are key molecules in the pathogenesis of fibrosis in multiple organs. To assess the potential utility of integrin αvβ3 imaging for idiopathic pulmonary fibrosis (IPF), we evaluated an 18F-FPP-RGD2 PET probe in a rat model of bleomycin-induced lung fibrosis.
Methods
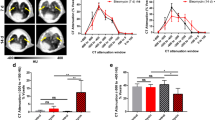
Pulmonary fibrosis was induced by single intratracheal instillation of bleomycin (3 mg/rat). Positron emission tomography (PET)/computerized tomography scans were performed 4 weeks after bleomycin administration using 18F-FPP-RGD2. Total distribution volume (VT) was estimated using one-tissue/two-compartment, two-tissue/three-compartment models, and Logan graphical analysis (Logan plot; t* = 30 min). Plasma-free fractions were estimated from images of the left ventricle. Correlation between Logan VT and lung pathology was assessed by Spearman’s rank correlation.
Results
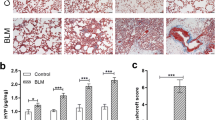
Histopathological evaluation demonstrated the development of fibrosis in IPF-model group. Integrin αv protein expression and lung radioactivity were higher in IPF-model group compared with control group. The lung radioactivity of 18F-FPP-RGD2 rapidly reached the peak after administration and then gradually decreased, whereas left ventricular radioactivity rapidly disappeared. Logan graphical analysis was found to be suitable for 18F-FPP-RGD2 kinetic analysis in the IPF-model lung. Logan VT values for 18F-FPP-RGD2 were significantly higher in IPF rats compared with control rats and strongly correlated with lung fibrosis, pathology, integrin αv protein expression, and oxygen partial pressure.
Conclusion
Our findings demonstrate that the integrin αvβ3 PET probe 18F-FPP-RGD2 can detect pathophysiological changes in lungs, including fibrosis accompanying upregulated integrin αv of IPF-model rats. These findings support the utility of 18F-FPP-RGD2 PET imaging for the pathophysiological evaluation of pulmonary fibrosis.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Gribbin J, Hubbard RB, Le Jeune I, Smith CJP, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax Thorax. 2006;61:980–5.
Ogura T, Taniguchi H, Azuma A, Inoue Y, Kondoh Y, Hasegawa Y, et al. Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J Eur Respir J. 2015;45:1382–92.
Raghu G. Pharmacotherapy for idiopathic pulmonary fibrosis: current landscape and future potential. Eur Respir Rev. Eur Respir Rev. 2017;26
Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States 2000 to 2011. Am J Respir Crit Care Med. 2016;193:1161–7.
Souza CA, Müller NL, Flint J, Wright JL, Churg A. Idiopathic pulmonary fibrosis: spectrum of high-resolution CT findings. AJR Am J Roentgenol. 2005;185:1531–9.
Hunninghake GW, Bridget Zimmerman M, Schwartz DA, King TE, Lynch J, Hegele R, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:193–6.
Lukey PT, Coello C, Gunn R, Parker C, Wilson FJ, Saleem A, et al. Clinical quantification of the integrin αvβ6 by [18F]FB-A20FMDV2 positron emission tomography in healthy and fibrotic human lung (PETAL Study). Eur J Nucl Med Mol Imaging: Springer; 2019.
Kimura RH, Wang L, Shen B, Huo L, Tummers W, Filipp FV, et al. Evaluation of integrin αvβ6 cystine knot PET tracers to detect cancer and idiopathic pulmonary fibrosis 2019 101. Nat Commun. 2019;101(10):1–18 (Nature Publishing Group).
Wuyts WA, Wijsenbeek M, Bondue B, Bouros D, Bresser P, RobaloCordeiro C, et al. Idiopathic Pulmonary Fibrosis: Best Practice in Monitoring and Managing a Relentless Fibrotic Disease. Respiration. 2020;99:73 (Karger Publishers).
Goodwin A, Jenkins G. Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans. 2009;37:849–54.
Henderson NC, Sheppard D. Integrin-mediated regulation of TGFβ in fibrosis. Biochim Biophys Acta. 2013;1832:891–6.
Schniering J, Benešová M, Brunner M, Haller S, Cohrs S, Frauenfelder T, et al. Visualisation of interstitial lung disease by molecular imaging of integrin α v β 3 and somatostatin receptor 2. Ann Rheum Dis. 2019;78:218–27.
Horan GS, Wood S, Ona V, Dan JL, Lukashev ME, Weinreb PH, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65.
Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, et al. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q). Am J Pathol Am J Pathol. 2009;174:1264–79.
John AE, Luckett JC, Tatler AL, Awais RO, Desai A, Habgood A, et al. Preclinical SPECT/CT imaging of αvβ6 integrins for molecular stratification of idiopathic pulmonary fibrosis. J Nucl Med J Nucl Med. 2013;54:2146–52.
Guo N, Lang L, Li W, Kiesewetter DO, Gao H, Niu G, et al. Quantitative analysis and comparison study of [18F]AlF-NOTA-PRGD2 [18F]FPPRGD2 and [68Ga]Ga-NOTA-PRGD2 using a reference tissue model. PLoS One. 2012;7:37506–14.
Hiroyama S, Rokugawa T, Ito M, Iimori H, Morita I, Maeda H, et al. Quantitative evaluation of hepatic integrin αvβ3 expression by positron emission tomography imaging using 18F-FPP-RGD2 in rats with non-alcoholic steatohepatitis. EJNMMI Res. 2020;10:118–29.
Kapp TG, Rechenmacher F, Neubauer S, Maltsev OV, Cavalcanti-Adam EA, Zarka R, et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci Rep. 2017;7:39805–17.
Haskali MB, Roselt PD, Karas JA, Noonan W, Wichmann CW, Katsifis A, et al. One-step radiosynthesis of 4-nitrophenyl 2-[(18) F]fluoropropionate ([(18) F]NFP); improved preparation of radiolabeled peptides for PET imaging. J Labelled Comp Radiopharm. 2013;56:726–30.
Jin ZH, Furukawa T, Sogawa C, Claron M, Aung W, Tsuji AB, et al. PET imaging and biodistribution analysis of the effects of succinylated gelatin combined with l-lysine on renal uptake and retention of 64Cu-cyclam-RAFT-c(-RGDfK-)4 in vivo. Eur J Pharm Biopharm Elsevier. 2014;86:478–86.
Bergeron M, Cadorette J, Tétrault MA, Beaudoin JF, Leroux JD, Fontaine R, et al. Imaging performance of LabPET APD-based digital PET scanners for pre-clinical research. Phys Med Biol Phys Med Biol. 2014;59:661–78.
Holman BF, Cuplov V, Millner L, Hutton BF, Maher TM, Groves AM, et al. Improved correction for the tissue fraction effect in lung PET / CT imaging. Phys Med Biol IOP Publishing. 2015;60:7387–402.
Lambrou T, Groves AM, Erlandsson K, Screaton N, Endozo R, Win T, et al. The importance of correction for tissue fraction effects in lung PET: Preliminary findings. Eur J Nucl Med Mol Imaging Springer. 2011;38:2238–46.
Akaike H. A New Look at the Statistical Model Identification. IEEE Trans Autom Control Springer. 1974;19:716–23.
Persson IM, Pettersson NF, Liu J, Håkansson HF, Örbom A, Zandt RI, et al. Longitudinal imaging using pet/ct with collagen-i pet-tracer and mri for assessment of fibrotic and inflammatory lesions in a rat lung injury model. J Clin Med. 2020;9:1–21.
Tashiro J, Elliot SJ, Gerth DJ, Xia X, Pereira-Simon S, Choi R, et al. Therapeutic benefits of young, but not old, adipose-derived mesenchymal stem cells in a chronic mouse model of bleomycin-induced pulmonary fibrosis. Transl Res Mosby. 2015;166:554–67.
Yousefi-Manesh H, Noori T, Asgardoon MH, Derakhshan MH, Tavangar SM, Sheibani M, et al. Protective effect of dapsone against bleomycin-induced lung fibrosis in rat. Exp Mol Pathol. 2022;124:104737–44.
Schniering J, Benešová M, Brunner M, Haller S, Cohrs S, Frauenfelder T, et al. Visualisation of interstitial lung disease by molecular imaging of integrin αvβ3 and somatostatin receptor 2. Ann Rheum Dis. 2019;78:218–27.
Lukey PT, Coello C, Gunn R, Parker C, Wilson FJ, Saleem A, et al. Clinical quantification of the integrin αvβ6 by [18 F]FB-A20FMDV2 positron emission tomography in healthy and fibrotic human lung (PETAL Study). Eur J Nucl Med Mol Imaging. 2020;47:967–79.
Chu KA, Yeh CC, Kuo FH, Lin WR, Hsu CW, Chen TH, et al. Comparison of reversal of rat pulmonary fibrosis of nintedanib, pirfenidone, and human umbilical mesenchymal stem cells from Wharton’s jelly. Stem Cell Res Ther BioMed Central Ltd. 2020;11:1–14.
Tanguy J, Goirand F, Bouchard A, Frenay J, Moreau M, Mothes C, et al. [18 F]FMISO PET/CT imaging of hypoxia as a non-invasive biomarker of disease progression and therapy efficacy in a preclinical model of pulmonary fibrosis: comparison with the [18 F]FDG PET/CT approach. Eur J Nucl Med Mol Imaging. 2021;48:3058–74.
Acknowledgements
We acknowledge Hirotada Murayama and Yumi Asano from Shionogi TechnoAdvance Research Co., Ltd. for preparation of the pathology specimens. We thank members of the Imaging Group at Shionogi & Co., Ltd. for their constructive discussion. We also thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This study was funded by Shionogi & Co., Ltd.
Author information
Authors and Affiliations
Contributions
SH performed the PET experiments, protein analyses, data analysis, and drafted the manuscript. KM performed data analysis and supervised the study. MI performed the PET experiments. HI synthesized the PET probe. MT performed the histological analysis. YN performed the preparation of IPF-model rats. ES supervised the study. KA coordinated and designed the study and drafted the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Shionogi & Co. Ltd. and Osaka University Graduate School of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hiroyama, S., Matsunaga, K., Ito, M. et al. Usefulness of 18F-FPP-RGD2 PET in pathophysiological evaluation of lung fibrosis using a bleomycin-induced rat model. Eur J Nucl Med Mol Imaging 49, 4358–4368 (2022). https://doi.org/10.1007/s00259-022-05908-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05908-4