Abstract
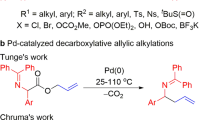
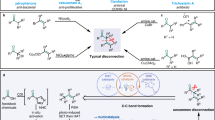
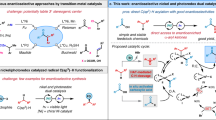
Aliphatic allylamines are widely used for the synthesis of diverse building blocks for agrochemicals and pharmaceuticals; there is therefore considerable interest in developing versatile and direct routes to aliphatic allylamines using common chemical feedstocks—olefins and amines. However, examples of such coupling reactions remain limited. It is even more challenging to achieve this goal with precise site control. Here we report that the combination of a photocatalyst and cobaloxime enables site-selective amination of olefins with secondary alkyl amines to afford allylic amines, eliminating the need for oxidants. This method is compatible with a broad scope of olefins and can be extended to achieve a site- and diastereoselective amination of terpenes. Mechanistic studies disclose that the reaction proceeds via a cobaloxime-promoted hydrogen atom transfer pathway to afford the product that results from cleavage of the stronger, primary allylic C–H bonds over other weaker allylic C–H bond options.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information, or from the authors on reasonable request.
References
Mayol-Llinas, J., Nelson, A., Farnaby, W. & Ayscough, A. Assessing molecular scaffolds for CNS drug discovery. Drug Discov. Today 22, 965–969 (2017).
Stütz, A. Allylamine derivatives—a new class of active substances in antifungal chemotherapy. Angew. Chem. Int. Ed. 26, 320–328 (1987).
Petranyi, G., Ryder, N. & Stutz, A. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science 224, 1239–1241 (1984).
Ramirez, T. A., Zhao, B. & Shi, Y. Recent advances in transition metal-catalyzed sp3 C–H amination adjacent to double bonds and carbonyl groups. Chem. Soc. Rev. 41, 931–942 (2012).
Georgopapadakou, N. H. & Walsh, T. J. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 40, 279–291 (1996).
Balfour, J. A. & Faulds, D. Terbinafine A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial mycoses. Drugs 43, 259–284 (1992).
Wang, J., Chen, Y., Ye, C., Qin, A. & Tang, B. Z. C(sp3)–H polyamination of internal alkynes toward regio- and stereoregular functional poly(allylic tertiary amine)s. Macromolecules 53, 3358–3369 (2020).
Shiratori, S. S. & Rubner, M. F. pH-Dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules 33, 4213–4219 (2000).
Johannsen, M. & Jørgensen, K. A. Allylic amination. Chem. Rev. 98, 1689–1708 (1998).
Bayeh, L., Le, P. Q. & Tambar, U. K. Catalytic allylic oxidation of internal alkenes to a multifunctional chiral building block. Nature 547, 196–200 (2017).
Teh, W. P., Obenschain, D. C., Black, B. M. & Michael, F. E. Catalytic metal-free allylic C–H amination of terpenoids. J. Am. Chem. Soc. 142, 16716–16722 (2020).
Liang, C. et al. Toward a synthetically useful stereoselective C–H amination of hydrocarbons. J. Am. Chem. Soc. 130, 343–350 (2008).
Harvey, M. E., Musaev, D. G. & Du Bois, J. A diruthenium catalyst for selective, intramolecular allylic C–H amination: reaction development and mechanistic insight gained through experiment and theory. J. Am. Chem. Soc. 133, 17207–17216 (2011).
Reed, S. A., Mazzotti, A. R. & White, M. C. A catalytic, Brønsted base strategy for intermolecular allylic C–H amination. J. Am. Chem. Soc. 131, 11701–11706 (2009).
Pattillo, C. C. et al. Aerobic linear allylic C–H amination: overcoming benzoquinone inhibition. J. Am. Chem. Soc. 138, 1265–1272 (2016).
Yin, G., Wu, Y. & Liu, G. Scope and mechanism of allylic C–H amination of terminal alkenes by the palladium/PhI(OPiv)2 catalyst system: insights into the effect of naphthoquinone. J. Am. Chem. Soc. 132, 11978–11987 (2010).
Lei, H. & Rovis, T. A site-selective amination catalyst discriminates between nearly identical C–H bonds of unsymmetrical disubstituted alkenes. Nat. Chem. 12, 725–731 (2020).
Burman, J. S. & Blakey, S. B. Regioselective intermolecular allylic C–H amination of disubstituted olefins via rhodium/π-allyl intermediates. Angew. Chem. Int. Ed. 56, 13666–13669 (2017).
Cheng, Q., Chen, J., Lin, S. & Ritter, T. Allylic amination of alkenes with iminothianthrenes to afford alkyl allylamines. J. Am. Chem. Soc. 142, 17287–17293 (2020).
Wang, D. J., Targos, K. & Wickens, Z. K. Electrochemical synthesis of allylic amines from terminal alkenes and secondary amines. J. Am. Chem. Soc. 143, 21503–21510 (2021).
Trowbridge, A., Walton, S. M. & Gaunt, M. J. New strategies for the transition-metal catalyzed synthesis of aliphatic amines. Chem. Rev. 120, 2613–2692 (2020).
Li, M.-L., Yu, J.-H., Li, Y.-H., Zhu, S.-F. & Zhou, Q.-L. Highly enantioselective carbene insertion into N–H bonds of aliphatic amines. Science 366, 990–994 (2019).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017).
Wang, H., Gao, X., Lv, Z., Abdelilah, T. & Lei, A. Recent advances in oxidative R(1)–H/R(2)–H cross-coupling with hydrogen evolution via photo-/electrochemistry. Chem. Rev. 119, 6769–6787 (2019).
Musacchio, A. J. et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science 355, 727–730 (2017).
Ruffoni, A. et al. Practical and regioselective amination of arenes using alkyl amines. Nat. Chem. 11, 426–433 (2019).
Ganley, J. M., Murray, P. R. D. & Knowles, R. R. Photocatalytic generation of aminium radical cations for C–N bond formation. ACS Catal. 10, 11712–11738 (2020).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Li, G., Han, A., Pulling, M. E., Estes, D. P. & Norton, J. R. Evidence for formation of a Co-H bond from (H2O)2Co(dmgBF2)2 under H2: application to radical cyclizations. J. Am. Chem. Soc. 134, 14662–14665 (2012).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
He, K.-H. et al. Acceptorless dehydrogenation of N-heterocycles by merging visible-light photoredox catalysis and cobalt catalysis. Angew. Chem. Int. Ed. 56, 3080–3084 (2017).
Rodrigalvarez, J. et al. Catalytic C(sp3)–H bond activation in tertiary alkylamines. Nat. Chem. 12, 76–81 (2020).
Avdagić, A., Gelo-Pujić, M. & Šunjić, V. Enantioselective chemoenzymatic synthesis of the S-enantiomer of the systemic fungicide fenpropimorph. Synthesis 1995, 1427–1431 (1995).
Vasseur, A., Bruffaerts, J. & Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016).
Burčul, F., Blažević, I., Radan, M. & Politeo, O. Terpenes, phenylpropanoids, sulfur and other essential oil constituents as inhibitors of cholinesterases. Curr. Med. Chem. 27, 4297–4343 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China 22031008 (A.L.) and Science Foundation of Wuhan 2020010601012192 (A.L.). We thank Y. Xi (UC Santa Barbara) for helpful discussions; W.L. (WHU) for the revision of manuscript; W. Kong (WHU) and X. Dong (WHU) for assistance with chiral HPLC analysis; and C. Bao (Taiwan Photon Source, TPS-44A), J. Chen (TPS-44A) and J. Lee (TPS-44A) for XAFS testing. X.Q. acknowledges the supercomputing system in the Supercomputing Center of Wuhan University.
Author information
Authors and Affiliations
Contributions
A.L. and S.W. conceived the work. S.W., Y.G., L.N and R.S. designed the experiments and analysed the data. S.W., Y.G., D.R., H.S. and X.L. performed the experiments. S.W., D.Y. and D.Z. contributed to the XAFS data. S.W. contributed to the EPR data. Z.L. and X.Q. contributed to the DFT calculations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Wujiong Xia and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Discussion, Tables 1–7 and Figs. 1–18.
Rights and permissions
About this article
Cite this article
Wang, S., Gao, Y., Liu, Z. et al. Site-selective amination towards tertiary aliphatic allylamines. Nat Catal 5, 642–651 (2022). https://doi.org/10.1038/s41929-022-00818-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00818-y
This article is cited by
-
Cobalt-catalysed allylic fluoroalkylation of terpenes
Nature Synthesis (2023)
-
A strategy for the site-selective allylic fluoroalkylation of terpenes
Nature Synthesis (2023)