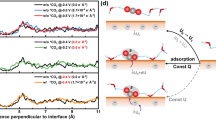
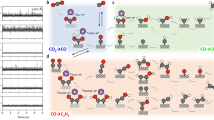
Abstract
Specifically adsorbed alkali metal cations on metal electrodes have been hypothesized to influence the reduction of CO2. However, experimental detection of these cations during CO2 reduction remains elusive. Herein, employing the asymmetric CH3 deformation band of tetramethylammonium as a vibrational probe of the aqueous electrolyte–polycrystalline Au interface, we monitored the displacement of specifically adsorbed tetramethylammonium by alkali metal cations. We found that the coverage of specifically adsorbed alkali metal cations during CO2-to-CO reduction follows the order Li+ < Na+ < K+ < Cs+ for the same bulk concentration. The alkali metal cations’ experimentally observed surface coverages correlate with their free energies of hydration. Furthermore, the rate of CO2-to-CO conversion increases with the coverage of specifically adsorbed alkali metal cations. Our observations suggest that the degree to which alkali metal cations undergo partial dehydration at the electrode–electrolyte interface plays a key role in their ability to promote CO2-to-CO reduction.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Representative data and extended datasets that support the findings reported in this study are available in the manuscript and the Supplementary Information. The data in the figures shown in the main text and DFT-calculated coordinates for the optimized geometries of the cations on Au(111) are provided in machine-readable formats as supplementary files. Additional data are available from the corresponding authors upon reasonable request.
References
Parsons, R. The electrical double layer: recent experimental and theoretical developments. Chem. Rev. 90, 813–826 (1990).
Dunwell, M., Yan, Y. & Xu, B. Understanding the influence of the electrochemical double-layer on heterogeneous electrochemical reactions. Curr. Opin. Chem. Eng. 20, 151–158 (2018).
Magnussen, O. M. & Groß, A. Toward an atomic-scale understanding of electrochemical interface structure and dynamics. J. Am. Chem. Soc. 141, 4777–4790 (2019).
Waegele, M. M., Gunathunge, C. M., Li, J. & Li, X. How cations affect the electric double layer and the rates and selectivity of electrocatalytic processes. J. Chem. Phys. 151, 160902 (2019).
Grahame, D. C. The electrical double layer and the theory of electrocapillarity. Chem. Rev. 41, 441–501 (1947).
Sorenson, S. A., Patrow, J. G. & Dawlaty, J. M. Solvation reaction field at the interface measured by vibrational sum frequency generation spectroscopy. J. Am. Chem. Soc. 139, 2369–2378 (2017).
Raberg, J. H. et al. Probing electric double-layer composition via in situ vibrational spectroscopy and molecular simulations. J. Phys. Chem. Lett. 10, 3381–3389 (2019).
Zhang, R. et al. Potential-dependent layering in the electrochemical double layer of water-in-salt electrolytes. ACS Appl. Energy Mater. 3, 8086–8094 (2020).
Xue, S., Garlyyev, B., Auer, A., Kunze-Liebhäuser, J. & Bandarenka, A. S. How the nature of the alkali metal cations influences the double-layer capacitance of Cu, Au, and Pt single-crystal electrodes. J. Phys. Chem. C 124, 12442–12447 (2020).
Zhang, Y. et al. Real-time characterization of the fine structure and dynamics of an electrical double layer at electrode–electrolyte interfaces. J. Phys. Chem. Lett. 12, 5279–5285 (2021).
Goldsmith, Z. K., Calegari Andrade, M. F. & Selloni, A. Effects of applied voltage on water at a gold electrode interface from ab initio molecular dynamics. Chem. Sci. 12, 5865–5873 (2021).
Ojha, K., Doblhoff-Dier, K. & Koper, M. T. M. Double-layer structure of the Pt(111)–aqueous electrolyte interface. Proc. Natl Acad. Sci. USA 119, e2116016119 (2022).
Herasymenko, P. & Šlendyk, I. Wasserstoffüberspannung und adsorption der ionen [hydrogen overpotential and adsorption of ions.]. Z. Phys. Chem. (N F) 149, 123–139 (1930).
Frumkin, A. N. Influence of cation adsorption on the kinetics of electrode processes. Trans. Faraday Soc. 55, 156–167 (1959).
Danilovic, N. et al. The effect of noncovalent interactions on the HOR, ORR, and HER on Ru, Ir, and Ru0.50Ir0.50 metal surfaces in alkaline environments. Electrocatalysis 3, 221–229 (2012).
Arán-Ais, R. M., Gao, D. & Roldan Cuenya, B. Structure- and electrolyte-sensitivity in CO2 electroreduction. Acc. Chem. Res. 51, 2906–2917 (2018).
Li, J. et al. Electrokinetic and in situ spectroscopic investigations of CO electrochemical reduction on copper. Nat. Commun. 12, 3264 (2021).
Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev. 109, 2209–2244 (2009).
McEnaney, J. M. et al. Electrolyte engineering for efficient electrochemical nitrate reduction to ammonia on a titanium electrode. ACS Sustain. Chem. Eng. 8, 2672–2681 (2020).
Yang, X., Wang, Y., Li, C. M. & Wang, D. Mechanisms of water oxidation on heterogeneous catalyst surfaces. Nano Res. 14, 3446–3457 (2021).
Huang, J., Li, M., Eslamibidgoli, M. J., Eikerling, M. & Groß, A. Cation overcrowding effect on the oxygen evolution reaction. JACS Au 1, 1752–1765 (2021).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Rao, R. R. et al. pH- and cation-dependent water oxidation on rutile RuO2(110). J. Phys. Chem. C 125, 8195–8207 (2021).
Strmcnik, D. et al. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat. Chem. 1, 466–472 (2009).
Feaster, J. T. et al. Understanding the influence of [EMIM]Cl on the suppression of the hydrogen evolution reaction on transition metal electrodes. Langmuir 33, 9464–9471 (2017).
Bhargava, S. S. et al. Exploring multivalent cations-based electrolytes for CO2 electroreduction. Electrochim. Acta 394, 139055 (2021).
Chen, L. D., Urushihara, M., Chan, K. & Nørskov, J. K. Electric field effects in electrochemical CO2 reduction. ACS Catal. 6, 7133–7139 (2016).
Akhade, S. A., McCrum, I. T. & Janik, M. J. The impact of specifically adsorbed ions on the copper-catalyzed electroreduction of CO2. J. Electrochem. Soc. 163, 477–484 (2016).
Gunathunge, C. M., Ovalle, V. J. & Waegele, M. M. Probing promoting effects of alkali cations on the reduction of CO at the aqueous electrolyte/copper interface. Phys. Chem. Chem. Phys. 19, 30166–30172 (2017).
Chen, X., McCrum, I. T., Schwarz, K. A., Janik, M. J. & Koper, M. T. M. Co-adsorption of cations as the cause of the apparent pH dependence of hydrogen adsorption on a stepped platinum single-crystal electrode. Angew. Chem. Int. Ed. 56, 15025–15029 (2017).
Pérez-Gallent, E., Marcandalli, G., Figueiredo, M. C., Calle-Vallejo, F. & Koper, M. T. M. Structure- and potential-dependent cation effects on CO reduction at copper single-crystal electrodes. J. Am. Chem. Soc. 139, 16412–16419 (2017).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Zhu, Q., Wallentine, S. K., Deng, G.-H., Rebstock, J. A. & Baker, L. R. The solvation-induced Onsager reaction field rather than the double-layer field controls CO2 reduction on gold. JACS Au 2, 472–482 (2022).
Liu, H., Liu, J. & Yang, B. Promotional role of a cation intermediate complex in C2 formation from electrochemical reduction of CO2 over cu. ACS Catal. 11, 12336–12343 (2021).
Fink, A. G. et al. Impact of alkali cation identity on the conversion of \({\mathrm{HCO}}_{3}^{-}\) to CO in bicarbonate electrolyzers. ChemElectroChem 8, 2094–2100 (2021)..
Singh, M. R., Kwon, Y., Lum, Y., Ager, J. W. & Bell, A. T. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 138, 13006–13012 (2016).
Ayemoba, O. & Cuesta, A. Spectroscopic evidence of size-dependent buffering of interfacial pH by cation hydrolysis during CO2 electroreduction. ACS Appl. Mater. Interfaces 9, 27377–27382 (2017).
Zhang, F. & Co, A. C. Direct evidence of local pH change and the role of alkali cation during CO2 electroreduction in aqueous media. Angew. Chem. Int. Ed. 58, 2–10 (2019).
Li, J., Li, X., Gunathunge, C. M. & Waegele, M. M. Hydrogen bonding steers the product selectivity of electrocatalytic CO reduction. Proc. Natl Acad. Sci. USA 116, 9220–9229 (2019).
Zhang, Z.-Q., Banerjee, S., Thoi, V. S. & Shoji Hall, A. Reorganization of interfacial water by an amphiphilic cationic surfactant promotes CO2 reduction. J. Phys. Chem. Lett. 11, 5457–5463 (2020).
Hussain, G. et al. How cations determine the interfacial potential profile: relevance for the CO2 reduction reaction. Electrochim. Acta 327, 135055 (2019).
Malkani, A. S. et al. Understanding the electric and nonelectric field components of the cation effect on the electrochemical CO reduction reaction. Sci. Adv. 6, eabd2569 (2020).
Wallentine, S., Bandaranayake, S., Biswas, S. & Baker, L. R. Direct observation of carbon dioxide electroreduction on gold: site blocking by the stern layer controls CO2 adsorption kinetics. J. Phys. Chem. Lett. 11, 8307–8313 (2020).
Mills, J. N., McCrum, I. T. & Janik, M. J. Alkali cation specific adsorption onto fcc(111) transition metal electrodes. Phys. Chem. Chem. Phys. 16, 13699–13707 (2014).
Matanovic, I., Atanassov, P., Garzon, F. & Henson, N. J. Density functional theory study of the alkali metal cation adsorption on Pt(111), Pt(100), and Pt(110) surfaces. ECS Trans. 61, 47–53 (2014).
McCrum, I. T. & Janik, M. J. pH and alkali cation effects on the Pt cyclic voltammogram explained using density functional theory. J. Phys. Chem. C. 120, 457–471 (2016).
Frank, D. G. et al. pH and potential dependence of the electrical double layer at well-defined electrode surfaces: Cs+ and Ca2+ ions at Pt(111) (\(2\sqrt{3}\times 2\sqrt{3}\))R30°-CN, Pt(111) (\(2\sqrt{3}\times 2\sqrt{3}\))R14°-CN, and Pt(111) (2 × 2)-SCN. Langmuir 1, 587–592 (1985).
Salaita, G. N. et al. Structure and composition of a platinum(111) surface as a function of pH and electrode potential in aqueous bromide solutions. Langmuir 2, 828–835 (1986).
Lucas, C. A., Thompson, P., Gründer, Y. & Markovic, N. M. The structure of the electrochemical double layer: Ag(111) in alkaline electrolyte. Electrochem. Commun. 13, 1205–1208 (2011).
Strmcnik, D. et al. Effects of Li+, K+, and Ba2+ cations on the ORR at model and high surface area Pt and Au surfaces in alkaline solutions. J. Phys. Chem. Lett. 2, 2733–2736 (2011).
Liu, Y., Kawaguchi, T., Pierce, M. S., Komanicky, V. & You, H. Layering and ordering in electrochemical double layers. J. Phys. Chem. Lett. 9, 1265–1271 (2018).
Kawaguchi, T., Liu, Y., Karapetrova, E. A., Komanicky, V. & You, H. In-situ to ex-situ in-plane structure evolution of stern layers on Pt(111) surface: surface X-ray scattering studies. J. Electroanal. Chem. 875, 114495 (2020).
Sarkar, S., Maitra, A., Banerjee, S., Thoi, V. S. & Dawlaty, J. M. Electric fields at metal–surfactant interfaces: a combined vibrational spectroscopy and capacitance study. J. Phys. Chem. B 124, 1311–1321 (2020).
Pennathur, A. K., Voegtle, M. J., Menachekanian, S. & Dawlaty, J. M. Strong propensity of ionic liquids in their aqueous solutions for an organic-modified metal surface. J. Phys. Chem. C 124, 7500–7507 (2020).
Voegtle, M. J. et al. Interfacial polarization and ionic structure at the ionic liquid–metal interface studied by vibrational spectroscopy and molecular dynamics simulations. J. Phys. Chem. C 125, 2741–2753 (2021).
Harmon, K. M., Gennick, I. & Madeira, S. L. Hydrogen bonding. iv. Correlation of infrared spectral properties with C–H ⋯ X hydrogen bonding and crystal habit in tetramethylammonium ion salts. J. Phys. Chem. 78, 2585–2591 (1974).
McCrum, I. T., Hickner, M. A. & Janik, M. J. Quaternary ammonium cation specific adsorption on platinum electrodes: a combined experimental and density functional theory study. J. Electrochem. Soc. 165, 114–121 (2018).
He, F. et al. Stability of quaternary akyl ammonium cations during the hydrogen evolution reduction: a differential electrochemical mass spectrometry study. J. Phys. Chem. C 125, 5715–5722 (2021).
Deng, Z. & Irish, D. E. Potential dependence of the orientation of (CH3)4N+ adsorbed on a silver electrode. a SERS investigation. J. Phys. Chem. 98, 9371–9373 (1994).
Trasatti, S. & Lust, E. In Modern Aspects of Electrochemistry (eds White, R. E. et al.) 1–215 (Springer, 1999).
Osawa, M. Dynamic processes in electrochemical reactions studied by surface-enhanced infrared absorption spectroscopy (SEIRAS). Bull. Chem. Soc. Jpn 70, 2861–2880 (1997).
Wuttig, A., Yaguchi, M., Motobayashi, K., Osawa, M. & Surendranath, Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl Acad. Sci. USA 113, 4585–4593 (2016).
Dong, Q., Zhang, X., He, D., Lang, C. & Wang, D. Role of H2O in CO2 electrochemical reduction as studied in a water-in-salt system. ACS Cent. Sci. 5, 1461–1467 (2019).
Zhang, B. A., Ozel, T., Elias, J. S., Costentin, C. & Nocera, D. G. Interplay of homogeneous reactions, mass transport, and kinetics in determining selectivity of the reduction of CO2 on gold electrodes. ACS Cent. Sci. 5, 1097–1105 (2019).
Ringe, S. et al. Double layer charging driven carbon dioxide adsorption limits the rate of electrochemical carbon dioxide reduction on gold. Nat. Commun. 11, 33 (2020).
Gambarotta, S., Arena, F., Floriani, C. & Zanazzi, P. F. Carbon dioxide fixation: bifunctional complexes containing acidic and basic sites working as reversible carriers. J. Am. Chem. Soc. 104, 5082–5092 (1982).
Benn, E. E., Gaskey, B. & Erlebacher, J. D. Suppression of hydrogen evolution by oxygen reduction in nanoporous electrocatalysts. J. Am. Chem. Soc. 139, 3663–3668 (2017).
Chen, W., Liao, L. W., Cai, J., Chen, Y.-X. & Stimming, U. Unraveling complex electrode processes by differential electrochemical mass spectrometry and the rotating ring-disk electrode technique. J. Phys. Chem. C 123, 29630–29637 (2019).
Bondue, C. J., Graf, M., Goyal, A. & Koper, M. T. M. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 reduction. J. Am. Chem. Soc. 143, 279–285 (2021).
Wuttig, A. & Surendranath, Y. Impurity ion complexation enhances carbon dioxide reduction catalysis. ACS Catal. 5, 4479–4484 (2015).
Shang, H. et al. Effect of surface ligands on gold nanocatalysts for CO2 reduction. Chem. Sci. 11, 12298–12306 (2020).
Mezzavilla, S., Horch, S., Stephens, I. E. L., Seger, B. & Chorkendorff, I. Structure sensitivity in the electrocatalytic reduction of CO2 with gold catalysts. Angew. Chem. Int. Ed. 58, 3774–3778 (2019).
Gunathunge, C. M., Li, J., Li, X. & Waegele, M. M. Surface-adsorbed CO as an infrared probe of electrocatalytic interfaces. ACS Catal. 10, 11700–11711 (2020).
Gunathunge, C. M., Li, J., Li, X., Hong, J. J. & Waegele, M. M. Revealing the predominant surface facets of rough Cu electrodes under electrochemical conditions. ACS Catal. 10, 6908–6923 (2020).
Dunwell, M., Yang, X., Yan, Y. & Xu, B. Potential routes and mitigation strategies for contamination in interfacial specific infrared spectroelectrochemical studies. J. Phys. Chem. C 122, 24658–24664 (2018).
Cave, E. R. et al. Electrochemical CO2 reduction on au surfaces: mechanistic aspects regarding the formation of major and minor products. Phys. Chem. Chem. Phys. 19, 15856–15863 (2017).
Marcus, Y. Ion Properties (Marcel Dekker, 1997).
Acknowledgements
This work was supported by a CAREER award (to M.M.W.) from the National Science Foundation (no. CHE-1847841). N.A. and M.J.J. acknowledge the National Science Foundation for support (award no. CHE-1665155).
Author information
Authors and Affiliations
Contributions
All authors discussed the results and commented on and revised the manuscript. M.M.W. and V.J.O. conceived and designed the experiments. V.J.O. conducted the experiments. Y.-S.H. participated in data collection. M.J.J. and N.A. contributed the DFT work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Yanwei Lum, Wenbin Cai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Tables 1–4 and Notes 1 and 2.
Supplementary Data 1
Coordinates for optimized geometries of cations on Au(111).
Supplementary Data 2
Machine-readable data shown in Figs. 1–7.
Rights and permissions
About this article
Cite this article
Ovalle, V.J., Hsu, YS., Agrawal, N. et al. Correlating hydration free energy and specific adsorption of alkali metal cations during CO2 electroreduction on Au. Nat Catal 5, 624–632 (2022). https://doi.org/10.1038/s41929-022-00816-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00816-0
This article is cited by
-
Pure-water-fed, electrocatalytic CO2 reduction to ethylene beyond 1,000 h stability at 10 A
Nature Energy (2024)
-
Electrochemical Carbon Dioxide Reduction in Acidic Media
Electrochemical Energy Reviews (2024)
-
Bridging the complexity gap in computational heterogeneous catalysis with machine learning
Nature Catalysis (2023)
-
Electrochemical carbon–carbon coupling with enhanced activity and racemate stereoselectivity by microenvironment regulation
Nature Communications (2023)
-
Direct time-resolved observation of surface-bound carbon dioxide radical anions on metallic nanocatalysts
Nature Communications (2023)