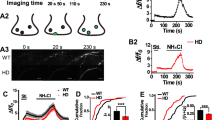
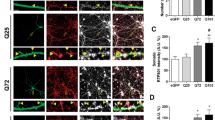
Abstract
Huntington's disease (HD) is a neurodegenerative disorder caused by a polyglutamine expansion in the protein huntingtin (HTT) [55]. While the final pathological consequence of HD is the neuronal cell death in the striatum region of the brain, it is still unclear how mutant HTT (mHTT) causes synaptic dysfunctions at the early stage and during the progression of HD. Here, we discovered that the basal activity of focal adhesion kinase (FAK) is severely reduced in a striatal HD cell line, a mouse model of HD, and the human post-mortem brains of HD patients. In addition, we observed with a FRET-based FAK biosensor [59] that neurotransmitter-induced FAK activation is decreased in HD striatal neurons. Total internal reflection fluorescence (TIRF) imaging revealed that the reduced FAK activity causes the impairment of focal adhesion (FA) dynamics, which further leads to the defect in filopodial dynamics causing the abnormally increased number of immature neurites in HD striatal neurons. Therefore, our results suggest that the decreased FAK and FA dynamics in HD impair the proper formation of neurites, which is crucial for normal synaptic functions [52]. We further investigated the molecular mechanism of FAK inhibition in HD and surprisingly discovered that mHTT strongly associates with phosphatidylinositol 4,5-biphosphate, altering its normal distribution at the plasma membrane, which is crucial for FAK activation [14, 60]. Therefore, our results provide a novel molecular mechanism of FAK inhibition in HD along with its pathological mechanism for synaptic dysfunctions during the progression of HD.







Similar content being viewed by others
References
Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431:805–810. https://doi.org/10.1038/nature02998
Barnat M, Capizzi M, Aparicio E, Boluda S, Wennagel D, Kacher R et al (2020) Huntington’s disease alters human neurodevelopment. Science 369:787–793. https://doi.org/10.1126/science.aax3338
Benarroch EE (2012) Effects of acetylcholine in the striatum. Recent Insights Therapeutic Implications Neurol 79:274–281. https://doi.org/10.1212/WNL.0b013e31825fe154
Berginski ME, Gomez SM (2013) The Focal Adhesion Analysis Server: a web tool for analyzing focal adhesion dynamics. F1000Res 2: 68 https://doi.org/10.12688/f1000research.2-68.v1
Burke KA, Kauffman KJ, Umbaugh CS, Frey SL, Legleiter J (2013) The interaction of polyglutamine peptides with lipid membranes is regulated by flanking sequences associated with huntingtin. J Biol Chem 288:14993–15005. https://doi.org/10.1074/jbc.M112.446237
Calandrella SO, Barrett KE, Keely SJ (2005) Transactivation of the epidermal growth factor receptor mediates muscarinic stimulation of focal adhesion kinase in intestinal epithelial cells. J Cell Physiol 203:103–110. https://doi.org/10.1002/jcp.20190
Carragher NO, Frame MC (2004) Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol 14:241–249. https://doi.org/10.1016/j.tcb.2004.03.011
Chacon MR, Fazzari P (2011) FAK: dynamic integration of guidance signals at the growth cone. Cell Adh Migr 5:52–55
Chacon MR, Navarro AI, Cuesto G, del Pino I, Scott R, Morales M et al (2012) Focal adhesion kinase regulates actin nucleation and neuronal filopodia formation during axonal growth. Development 139:3200–3210. https://doi.org/10.1242/dev.080564
DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP et al (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277:1990–1993. https://doi.org/10.1126/science.277.5334.1990
Fiala JC, Feinberg M, Popov V, Harris KM (1998) Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci 18:8900–8911
Fiala JC, Spacek J, Harris KM (2002) Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev 39:29–54. https://doi.org/10.1016/s0165-0173(02)00158-3
Fischer RS, Lam PY, Huttenlocher A, Waterman CM (2019) Filopodia and focal adhesions: an integrated system driving branching morphogenesis in neuronal pathfinding and angiogenesis. Dev Biol 451:86–95. https://doi.org/10.1016/j.ydbio.2018.08.015
Goni GM, Epifano C, Boskovic J, Camacho-Artacho M, Zhou J, Bronowska A et al (2014) Phosphatidylinositol 4,5-bisphosphate triggers activation of focal adhesion kinase by inducing clustering and conformational changes. Proc Natl Acad Sci USA 111:E3177-3186. https://doi.org/10.1073/pnas.1317022111
Gunawardena S, Her LS, Brusch RG, Laymon RA, Niesman IR, Gordesky-Gold B et al (2003) Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 40:25–40. https://doi.org/10.1016/s0896-6273(03)00594-4
Gupton SL, Gertler FB (2010) Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell 18:725–736. https://doi.org/10.1016/j.devcel.2010.02.017
Heckman CA, Plummer HK 3rd (2013) Filopodia as sensors. Cell Signal 25:2298–2311. https://doi.org/10.1016/j.cellsig.2013.07.006
Hickman RA, Faust PL, Rosenblum MK, Marder K, Mehler MF et al (2021) Developmental malformations in Huntington disease: neuropathologic evidence of focal neuronal migration defects in a subset of adult brains. Acta Neuropathol 141:399–413. https://doi.org/10.1007/s00401-021-02269-4
Hu YL, Lu S, Szeto KW, Sun J, Wang Y, Lasheras JC et al (2014) FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci Rep 4:6024. https://doi.org/10.1038/srep06024
Hwang YJ, Han D, Kim KY, Min SJ, Kowall NW, Yang L et al (2014) ESET methylates UBF at K232/254 and regulates nucleolar heterochromatin plasticity and rDNA transcription. Nucleic Acids Res 42:1628–1643. https://doi.org/10.1093/nar/gkt1041
Hyeon SJ, Park J, Yoo J, Kim SH, Hwang YJ, Kim SC et al (2021) Dysfunction of X-linked inhibitor of apoptosis protein (XIAP) triggers neuropathological processes via altered p53 activity in Huntington’s disease. Prog Neurobiol 204:102110. https://doi.org/10.1016/j.pneurobio.2021.102110
Jacquemet G, Hamidi H, Ivaska J (2015) Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr Opin Cell Biol 36:23–31. https://doi.org/10.1016/j.ceb.2015.06.007
Jacquemet G, Stubb A, Saup R, Miihkinen M, Kremneva E, Hamidi H et al (2019) Filopodome Mapping Identifies p130Cas as a Mechanosensitive Regulator of Filopodia Stability. Curr Biol 29(202–216):e207. https://doi.org/10.1016/j.cub.2018.11.053
Jontes JD, Smith SJ (2000) Filopodia, spines, and the generation of synaptic diversity. Neuron 27:11–14. https://doi.org/10.1016/s0896-6273(00)00003-9
Kegel-Gleason KB (2013) Huntingtin interactions with membrane phospholipids: strategic targets for therapeutic intervention? J Huntingtons Dis 2:239–250. https://doi.org/10.3233/JHD-130068
Kegel KB, Meloni AR, Yi Y, Kim YJ, Doyle E, Cuiffo BG et al (2002) Huntingtin is present in the nucleus, interacts with the transcriptional corepressor C-terminal binding protein, and represses transcription. J Biol Chem 277:7466–7476. https://doi.org/10.1074/jbc.M103946200
Kegel KB, Sapp E, Alexander J, Valencia A, Reeves P, Li X et al (2009) Polyglutamine expansion in huntingtin alters its interaction with phospholipids. J Neurochem 110:1585–1597. https://doi.org/10.1111/j.1471-4159.2009.06255.x
Kegel KB, Sapp E, Yoder J, Cuiffo B, Sobin L, Kim YJ et al (2005) Huntingtin associates with acidic phospholipids at the plasma membrane. J Biol Chem 280:36464–36473. https://doi.org/10.1074/jbc.M503672200
Khan RI, Yazawa T, Anisuzzaman AS, Semba S, Ma Y, Uwada J et al (2014) Activation of focal adhesion kinase via M1 muscarinic acetylcholine receptor is required in restitution of intestinal barrier function after epithelial injury. Biochim Biophys Acta 1842:635–645. https://doi.org/10.1016/j.bbadis.2013.12.007
Kim D, Jeon J, Cheong E, Kim DJ, Ryu H, Seo H et al (2016) Neuroanatomical visualization of the impaired striatal connectivity in huntington’s disease mouse model. Mol Neurobiol 53:2276–2286. https://doi.org/10.1007/s12035-015-9214-2
Kuemmerle S, Gutekunst CA, Klein AM, Li XJ, Li SH, Beal MF et al (1999) Huntington aggregates may not predict neuronal death in Huntington’s disease. Ann Neurol 46:842–849
Kulkarni VA, Firestein BL (2012) The dendritic tree and brain disorders. Mol Cell Neurosci 50:10–20. https://doi.org/10.1016/j.mcn.2012.03.005
Kwan W, Trager U, Davalos D, Chou A, Bouchard J, Andre R et al (2012) Mutant huntingtin impairs immune cell migration in Huntington disease. J Clin Invest 122:4737–4747. https://doi.org/10.1172/JCI64484
Labbadia J, Morimoto RI (2013) Huntington’s disease: underlying molecular mechanisms and emerging concepts. Trends Biochem Sci 38:378–385. https://doi.org/10.1016/j.tibs.2013.05.003
Lee J, Hong YK, Jeon GS, Hwang YJ, Kim KY, Seong KH et al (2012) ATRX induction by mutant huntingtin via Cdx2 modulates heterochromatin condensation and pathology in Huntington’s disease. Cell Death Differ 19:1109–1116. https://doi.org/10.1038/cdd.2011.196
Lee J, Hwang YJ, Kim Y, Lee MY, Hyeon SJ, Lee S et al (2017) Remodeling of heterochromatin structure slows neuropathological progression and prolongs survival in an animal model of Huntington’s disease. Acta Neuropathol 134:729–748. https://doi.org/10.1007/s00401-017-1732-8
Lee J, Hwang YJ, Shin JY, Lee WC, Wie J, Kim KY et al (2013) Epigenetic regulation of cholinergic receptor M1 (CHRM1) by histone H3K9me3 impairs Ca(2+) signaling in Huntington’s disease. Acta Neuropathol 125:727–739. https://doi.org/10.1007/s00401-013-1103-z
Li H, Li SH, Johnston H, Shelbourne PF, Li XJ (2000) Amino-terminal fragments of mutant huntingtin show selective accumulation in striatal neurons and synaptic toxicity. Nat Genet 25:385–389. https://doi.org/10.1038/78054
Li H, Li SH, Yu ZX, Shelbourne P, Li XJ (2001) Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. J Neurosci 21:8473–8481
Li JY, Conforti L (2013) Axonopathy in Huntington’s disease. Exp Neurol 246:62–71. https://doi.org/10.1016/j.expneurol.2012.08.010
Li SH, Li XJ (2004) Huntingtin-protein interactions and the pathogenesis of Huntington’s disease. Trends Genet 20:146–154. https://doi.org/10.1016/j.tig.2004.01.008
Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ (2007) Structural basis for the autoinhibition of focal adhesion kinase. Cell 129:1177–1187. https://doi.org/10.1016/j.cell.2007.05.041
Lin YC, Koleske AJ (2010) Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci 33:349–378. https://doi.org/10.1146/annurev-neuro-060909-153204
Maat-Schieman ML, Dorsman JC, Smoor MA, Siesling S, Van Duinen SG, Verschuuren JJ et al (1999) Distribution of inclusions in neuronal nuclei and dystrophic neurites in Huntington disease brain. J Neuropathol Exp Neurol 58:129–137. https://doi.org/10.1097/00005072-199902000-00003
Mattila PK, Lappalainen P (2008) Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 9:446–454. https://doi.org/10.1038/nrm2406
Milnerwood AJ, Raymond LA (2010) Early synaptic pathophysiology in neurodegeneration: insights from Huntington’s disease. Trends Neurosci 33:513–523. https://doi.org/10.1016/j.tins.2010.08.002
Mitra SK, Hanson DA, Schlaepfer DD (2005) Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6:56–68. https://doi.org/10.1038/nrm1549
Mitra SK, Schlaepfer DD (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18:516–523. https://doi.org/10.1016/j.ceb.2006.08.011
Moeller ML, Shi Y, Reichardt LF, Ethell IM (2006) EphB receptors regulate dendritic spine morphogenesis through the recruitment/phosphorylation of focal adhesion kinase and RhoA activation. J Biol Chem 281:1587–1598. https://doi.org/10.1074/jbc.M511756200
Murmu RP, Li W, Holtmaat A, Li JY (2013) Dendritic spine instability leads to progressive neocortical spine loss in a mouse model of Huntington’s disease. J Neurosci 33:12997–13009. https://doi.org/10.1523/JNEUROSCI.5284-12.2013
Murphy KP, Carter RJ, Lione LA, Mangiarini L, Mahal A, Bates GP et al (2000) Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington’s disease mutation. J Neurosci 20:5115–5123
Navarro AI, Rico B (2014) Focal adhesion kinase function in neuronal development. Curr Opin Neurobiol 27:89–95. https://doi.org/10.1016/j.conb.2014.03.002
Renaudin A, Lehmann M, Girault J, McKerracher L (1999) Organization of point contacts in neuronal growth cones. J Neurosci Res 55:458–471. https://doi.org/10.1002/(SICI)1097-4547(19990215)55:4%3c458::AID-JNR6%3e3.0.CO;2-D
Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF (2004) Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci 7:1059–1069. https://doi.org/10.1038/nn1317
Ross CA, Tabrizi SJ (2011) Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 10:83–98. https://doi.org/10.1016/s1474-4422(10)70245-3
Sainath R, Gallo G (2015) Cytoskeletal and signaling mechanisms of neurite formation. Cell Tissue Res 359:267–278. https://doi.org/10.1007/s00441-014-1955-0
Saudou F, Finkbeiner S, Devys D, Greenberg ME (1998) Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95:55–66. https://doi.org/10.1016/s0092-8674(00)81782-1
Saudou F, Humbert S (2016) The Biology of Huntingtin. Neuron 89:910–926. https://doi.org/10.1016/j.neuron.2016.02.003
Seong J, Ouyang M, Kim T, Sun J, Wen PC, Lu S et al (2011) Detection of focal adhesion kinase activation at membrane microdomains by fluorescence resonance energy transfer. Nat Commun 2:406. https://doi.org/10.1038/ncomms1414
Seong J, Tajik A, Sun J, Guan JL, Humphries MJ, Craig SE et al (2013) Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proc Natl Acad Sci USA 110:19372–19377. https://doi.org/10.1073/pnas.1307405110
Shen K, Cowan CW (2010) Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol 2:a001842. https://doi.org/10.1101/cshperspect.a001842
Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM (2009) Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci 29:8129–8142. https://doi.org/10.1523/JNEUROSCI.4681-08.2009
Stauffer TP, Ahn S, Meyer T (1998) Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol 8:343–346. https://doi.org/10.1016/s0960-9822(98)70135-6
Szebenyi G, Morfini GA, Babcock A, Gould M, Selkoe K, Stenoien DL et al (2003) Neuropathogenic forms of huntingtin and androgen receptor inhibit fast axonal transport. Neuron 40:41–52. https://doi.org/10.1016/s0896-6273(03)00569-5
Tao M, Pandey NK, Barnes R, Han S, Langen R (2019) Structure of Membrane-Bound Huntingtin Exon 1 Reveals Membrane Interaction and Aggregation Mechanisms. Structure 27(1570–1580):e1574. https://doi.org/10.1016/j.str.2019.08.003
Tomar A, Schlaepfer DD (2009) Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol 21:676–683. https://doi.org/10.1016/j.ceb.2009.05.006
Tourette C, Li B, Bell R, O’Hare S, Kaltenbach LS, Mooney SD et al (2014) A large scale Huntingtin protein interaction network implicates Rho GTPase signaling pathways in Huntington disease. J Biol Chem 289:6709–6726. https://doi.org/10.1074/jbc.M113.523696
Tousley A, Iuliano M, Weisman E, Sapp E, Richardson H, Vodicka P et al (2019) Huntingtin associates with the actin cytoskeleton and alpha-actinin isoforms to influence stimulus dependent morphology changes. PLoS ONE 14:e0212337. https://doi.org/10.1371/journal.pone.0212337
Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F et al (2000) Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet 9:2799–2809. https://doi.org/10.1093/hmg/9.19.2799
Turner CE (2000) Paxillin and focal adhesion signalling. Nat Cell Biol 2:E231-236. https://doi.org/10.1038/35046659
Usdin MT, Shelbourne PF, Myers RM, Madison DV (1999) Impaired synaptic plasticity in mice carrying the Huntington’s disease mutation. Hum Mol Genet 8:839–846. https://doi.org/10.1093/hmg/8.5.839
Valiente M, Ciceri G, Rico B, Marin O (2011) Focal adhesion kinase modulates radial glia-dependent neuronal migration through connexin-26. J Neurosci 31:11678–11691. https://doi.org/10.1523/JNEUROSCI.2678-11.2011
Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577. https://doi.org/10.1097/00005072-198511000-00003
Ziv NE, Garner CC (2004) Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci 5:385–399. https://doi.org/10.1038/nrn1370
Acknowledgements
This work was supported in part by National Research Foundation of Korea grant 2021R1A2C1093429, KIST Institutional grant 2E31523, and Samsung Research Funding & Incubation Center of Samsung Electronics under Project Number SRFC-TC2003-02 (J.S.). This work was also supported by NIH grant R01NS109537 (J.L.), National Research Foundation of Korea grant NRF-2020M3E5D9079742, and KIST Institutional grant 2E31505 (H.R.).
Author information
Authors and Affiliations
Contributions
JS and HR designed research; HNL, SJH, HK, KMS, YK, JJ performed experiments; JS, HR, HNL, SJH, JL, YW analyzed data; JS, HR, HNL wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 8323 KB)
Supplementary file2 (MP4 1491 KB)
Supplementary file4 (MP4 328 KB)
Supplementary file5 (MP4 1150 KB)
Rights and permissions
About this article
Cite this article
Lee, H.N., Hyeon, S.J., Kim, H. et al. Decreased FAK activity and focal adhesion dynamics impair proper neurite formation of medium spiny neurons in Huntington's disease. Acta Neuropathol 144, 521–536 (2022). https://doi.org/10.1007/s00401-022-02462-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02462-z