Abstract
Introduction
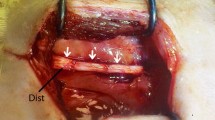
Given that denervation atrophy often occurs in muscle after peripheral nerve injury, the effects of injections of human adipose-derived stem cells (hADSCs) and platelet-rich plasma (PRP) into muscle after peripheral nerve injury were examined.
Methods
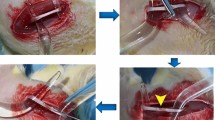
hADSCs were isolated from human subcutaneous fat tissue, and PRP was prepared from rat whole blood before injection into a rat sciatic nerve injury model. Muscle atrophy was evaluated by quantitating the gross musculature and muscle fiber area and walking footprint analysis.
Results
At 4 weeks post-surgery, there were significant differences in the sciatic functional index between experimental (injected with hADSCs, PRP, or combined hADSCs + PRP) and non-operated groups (p < 0.0001), but no significant differences were observed between the different treatment groups (p > 0.05). Post hoc Bonferroni tests also showed significant differences in the wet muscle weight ratios of hADSC, PRP, and combined groups compared to PBS group. The gastrocnemius muscle fiber area was larger in hADSC group and the combined group compared to PBS group at 4 weeks post-surgery.
Conclusion
The injection of hADSCs delays muscular atrophy after sciatic nerve injury in rats; thus, hADSCs are a promising alternative for regenerating atrophied muscle.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Yoshioka, N. (2020). Hypoglossal-facial side-to-end neurorrhaphy with concomitant masseteric-zygomatic nerve branch coaptation and muscle transfer for facial reanimation: technique and case report. Operative Neurosurgery (Hagerstown), 19(3), E230–E235.
Tabata, S., Aizawa, M., Kinoshita, M., Ito, Y., Kawamura, Y., Takebe, M., Pan, W., & Sakuma, K. (2019). The influence of isoflavone for denervation-induced muscle atrophy. European Journal Nutrition, 58(1), 291–300.
Choi, W. H., Jang, Y. J., Son, H. J., Ahn, J., Jung, C. H., & Ha, T. Y. (2018). Apigenin inhibits sciatic nerve denervation-induced muscle atrophy. Muscle & Nerve, 58(2), 314–318.
Bueno, C. R. S., Pereira, M., Favaretto, I. A. J., Bortoluci, C. H. F., Santos, T., Dias, D. V., Dare, L. R., & Rosa, G. M. J. (2017). Electrical stimulation attenuates morphological alterations and prevents atrophy of the denervated cranial tibial muscle. Einstein (Sao Paulo), 15(1), 71–76.
Su, Z., Hu, L., Cheng, J., Klein, J. D., Hassounah, F., Cai, H., Li, M., Wang, H. & Wang, X. H. (2016). Acupuncture plus low-frequency electrical stimulation (Acu-LFES) attenuates denervation-induced muscle atrophy. Journal of Applied Physiology, 120(4), 426–436.
Sowa, Y., Imura, T., Numajiri, T., Nishino, K., & Fushiki, S. (2012). Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells and Development, 21(11), 1852–1862.
Fairbairn, N. G., Meppelink, A. M., Ng-Glazier, J., Randolph, M. A., & Winograd, J. M. (2015). Augmenting peripheral nerve regeneration using stem cells: A review of current opinion. World Journal of Stem Cells, 7(1), 11–26.
Gimble, J. M., Bunnell, B. A., Chiu, E. S., & Guilak, F. (2011). Concise review: adipose-derived stromal vascular fraction cells and stem cells: let’s not get lost in translation. Stem Cells, 29(5), 749–754.
Minteer, D., Marra, K. G., & Rubin, J. P. (2013). Adipose-derived mesenchymal stem cells: biology and potential applications. Advances in Biochemical Engineering/Biotechnology, 129, 59–71.
Abdul Halim, N. S. S., Yahaya, B. H., & Lian, J. (2022). Therapeutic potential of adipose-derived stem cells in the treatment of pulmonary diseases. Current Stem Cell Research & Therapy, 17(2), 103–112.
Schilling, B. K., Schusterman, 2nd, M. A., Kim, D. Y., Repko, A. J., Klett, K. C., Christ, G. J., & Marra, K. G. (2019). Adipose-derived stem cells delay muscle atrophy after peripheral nerve injury in the rodent model. Muscle & Nerve, 59(5), 603–610.
Edalatmanesh, M. A., Bahrami, A. R., Hosseini, E., Hosseini, M., & Khatamsaz, S. (2011). Neuroprotective effects of mesenchymal stem cell transplantation in animal model of cerebellar degeneration. Neurological Research, 33(9), 913–920.
Haddad-Mashadrizeh, A., Bahrami, A. R., Matin, M. M., Edalatmanesh, M. A., Zomorodipour, A., Fallah, A., Gardaneh, M., Ahmadian Kia, N., & Sanjarmoosavi, N. (2013). Evidence for crossing the blood barrier of adult rat brain by human adipose-derived mesenchymal stromal cells during a 6-month period of post-transplantation. Cytotherapy, 15(8), 951–960.
Mohamed-Ahmed, S., Fristad, I., Lie, S. A., Suliman, S., Mustafa, K., Vindenes, H., & Idris, S. B. (2018). Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Research & Therapy, 9(1), 168.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D., & Horwitz, E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317.
Inserra, M. M., Bloch, D. A., & Terris, D. J. (1998). Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery, 18(2), 119–124.
Wu, R., Wang, L., Chen, F., Huang, Y., Shi, J., Zhu, X., Ding, Y., & Zhang, X. (2016). Evaluation of artificial nerve conduit and autografts in peripheral nerve repair in the rat model of sciatic nerve injury. Neurological Research, 38(5), 461–466.
Asami, Y., Aizawa, M., Kinoshita, M., Ishikawa, J. & Sakuma, K. (2018). Resveratrol attenuates denervation-induced muscle atrophy due to the blockade of atrogin-1 and p62 accumulation. International Journal of Medical Sciences, 15(6), 628–637.
Lala-Tabbert, N., Lejmi-Mrad, R., Timusk, K., Fukano, M., Holbrook, J., St-Jean, M., LaCasse, E. C., & Korneluk, R. G. (2019). Targeted ablation of the cellular inhibitor of apoptosis 1 (cIAP1) attenuates denervation-induced skeletal muscle atrophy. Skeletal Muscle, 9(1), 13.
Park, J. U., & Kwon, S. T. (2017). Potential of autologous adipose-derived stem cells to regenerate atrophied muscle in a rat model. Wound Repair and Regeneration, 25(6), 944–955.
Chen, H. S., Su, Y. T., Chan, T. M., Su, Y. J., Syu, W. S., Harn, H. J., Lin, S. Z., & Chiu, S. C. (2015). Human adipose-derived stem cells accelerate the restoration of tensile strength of tendon and alleviate the progression of rotator cuff injury in a rat model. Cell Transplantation, 24(3), 509–520.
Siregar, S., Sasongko Noegroho, B., Adriansjah, R., Mustafa, A. & Wijayanti, Z. (2021). Intratesticular human adipose-derived stem cell (hADSC) transplantation decreased oxidative stress in testicular torsion model of wistar rat. Research and Reports in Urology, 13, 1–8.
Zhang, M., Park, G., Zhou, B. & Luo, D. (2018). Applications and efficacy of platelet-rich plasma in dermatology: A clinical review. Journal of Cosmetic Dermatology, 17(5), 660–665.
Garbin, L. C., & Olver, C. S. (2020). Platelet-rich products and their application to osteoarthritis. Journal of Equine Veterinary Science, 86, 102820. https://doi.org/10.1016/j.jevs.2019.102820.
Chou, T. M., Chang, H. P. & Wang, J. C. (2020). Autologous platelet concentrates in maxillofacial regenerative therapy. The Kaohsiung Journal of Medical Sciences, 36(5), 305–310.
Andia, I., & Abate, M. (2013). Platelet-rich plasma: underlying biology and clinical correlates. Regenerative Medicine, 8(5), 645–658.
Mussano, F., Genova, T., Munaron, L., Petrillo, S., Erovigni, F., & Carossa, S. (2016). Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets, 27(5), 467–471.
Giusti, I., D’Ascenzo, S., Macchiarelli, G., & Dolo, V. (2020). In vitro evidence supporting applications of platelet derivatives in regenerative medicine. Blood Transfusion, 18(2), 117–129.
Emer, J. (2019). Platelet-rich plasma (PRP): current applications in dermatology. Skin Therapy Letter, 24(5), 1–6.
Wu, C. C., Chen, W. H., Zao, B., Lai, P. L., Lin, T. C., Lo, H. Y., Shieh, Y. H., Wu, C. H., & Deng, W. P. (2011). Regenerative potentials of platelet-rich plasma enhanced by collagen in retrieving pro-inflammatory cytokine-inhibited chondrogenesis. Biomaterials, 32(25), 5847–5854.
Funding
This study was supported by the Discipline Construction Project of Peking Union Medical College (201920200401) and CAMS Innovation Fund for Medical Sciences (2021-I2M-1-068).
Author information
Authors and Affiliations
Contributions
Q.S. performed the experiments, analyzed the data, and wrote the manuscript. M.N., W.W., C.S., W.Q., and L.Y. designed the research, analyzed the data, and contributed to the writing of the manuscript. Y.Z. supervised the study.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethical approval and consent to participate
All experiments conducted in this study were reviewed and approved by the Local Animal Ethics Committee (No. 202003003). Using human adipose tissue was approved by the Ethics Committee of Plastic Surgery Hospital (No. 2150019022). All methods in this study were conducted following relevant guidelines and regulations. All methods are reported in this study following ARRIVE guidelines for the reporting of animal experiments.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qu, S., Ma, N., Wang, W. et al. Human Adipose-Derived Stem Cells Delay Muscular Atrophy after Peripheral Nerve Injury in Rats. Cell Biochem Biophys 80, 555–562 (2022). https://doi.org/10.1007/s12013-022-01082-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-022-01082-4